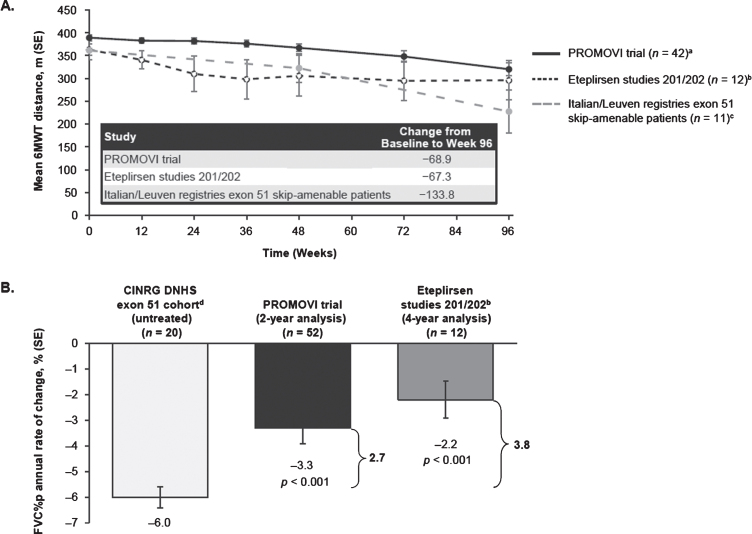
Fig. 3.
Post-hoc analysis: Mean change from baseline to Week 96 in 6MWT (A), and FVC%p (B) in eteplirsen-treated patients and matched comparisons. Abbreviations: FVC%p = percent predicted forced vital capacity; 6MWT = 6-minute walk test; SE = standard error. aAt Weeks 12, 72, and 96 (n = 41). One patient did not have a value at Week 12, but had at later visits. Another patient withdrew after Week 48. bEteplirsen studies 201 (NCT01396239) [24] and 202 (NCT01540409) [28]. cItalian DMD Telethon Registry [12, 25, 26] and the Leuven NMRC Registry [27]. dUntreated patients in the CINRG DNHS exon 51 cohort (age 10–18 years) [11].