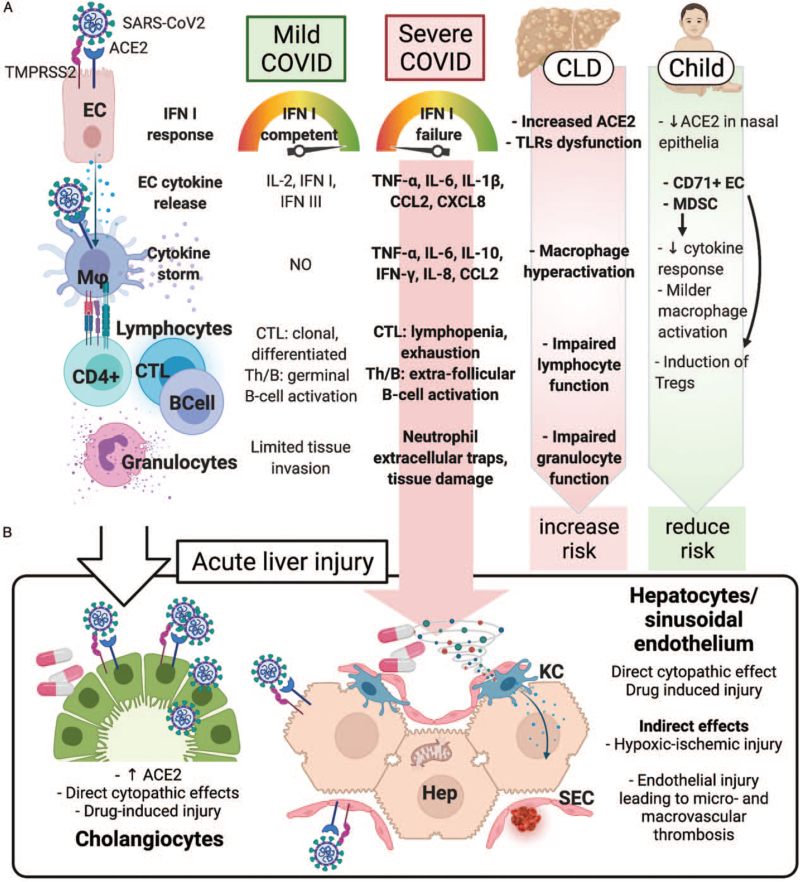
FIGURE 1.
Pathophysiology of the liver injury in SARS-CoV2 infection. (A) Immune pathophysiology of mild and severe COVID-19. In severe cases, viral and host factors may result in an inhibited IFN type I response, resulting in a proinflammatory cytokines and chemokines production. This milieu causes lung recruitment of macrophages and T cells, responsible for a cytokine storm, causing further neutrophil recruitment and NETosis, ultimately leading to tissue injury, diverted adaptive immune response, systemic inflammation, and organ dysfunction, including liver. In children, the detrimental role of a preexisting chronic liver disease (due to increased hepatocyte ACE2 expression, innate and adaptive immune dysfunction, macrophage hyperactivation) is counterbalanced by the immunotolerant phenotype associated with the young age. (B) Acute liver injury during SARS-CoV2 infection can occur via direct and indirect mechanisms. The systemic inflammatory cytokine milieu causes sinusoidal endothelial activation, with subsequent thrombosis and hypoxic-ischemic injury. Direct cytopathic effects, and drug-induced injury are also possible especially in cholangiocytes. ACE2 = angiotensin-converting enzyme 2; CCL2 = C–C motif chemokine ligand 2; CD4+ = CD4+ lymphocyte; CD71+ EC = CD71+ erythroid precursors; CLD = chronic liver disease; CTL = cytotoxic T cell; CXCL8 = C–X–C motif chemokine ligand 8; EC = epithelial (respiratory) cell; Hep = hepatocyte; IFN = interferon; IL-2, -6, -8, -10 = interleukin-2, -6, -8, -10; KC = Kupffer cell; Mϕ = macrophage; MDSC = myeloid-derived suppressor cells; SEC = sinusoidal endothelial cell; Th = helper T cell; TLRs = Toll-like receptors; TMPRSS2 = transmembrane protease, serine 2; TNF-α = tumor necrosis factor-α.