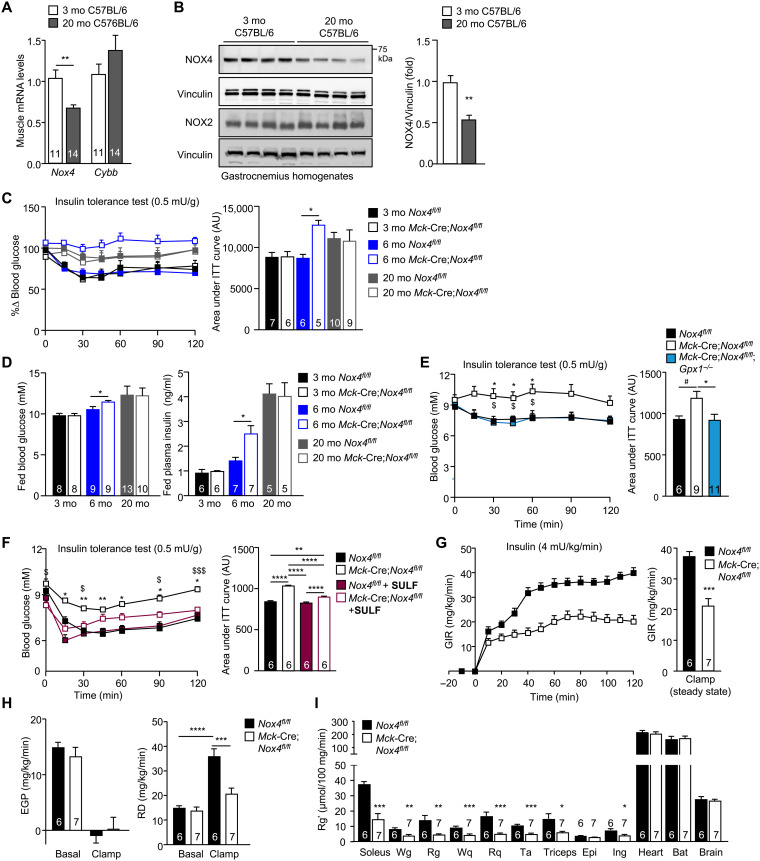
Fig. 7. NOX4-derived H2O2 in skeletal muscle is essential for attenuating the age-associated development of insulin resistance.
(A) Gastrocnemius muscle Nox4 and Cybb (encoding NOX2) expression in 3- versus 20-month-old chow-fed C57BL/6 male mice assessed by qPCR. (B) Gastrocnemius muscle NOX4 and NOX2 proteins in 3- versus 20-month-old chow-fed C57BL/6 male mice were assessed by immunoblotting. (C) Insulin tolerance tests (ITTs; 0.5 mU/g) or (D) fed (satiated) blood glucose and plasma insulin levels in 3-, 6-, and 20-month-old Nox4fl/fl and Mck-Cre;Nox4fl/fl chow-fed male mice; areas under ITT curves were determined, and arbitrary units (AU) are shown. (E) ITTs in 6-month-old Nox4fl/fl, Mck-Cre;Nox4fl/fl, and Mck-Cre;Nox4fl/fl;Gpx1−/− chow-fed (4.8% fat) male mice. (F) Six-month-old male Nox4fl/fl and Mck-Cre;Nox4fl/fl chow-fed male mice were administered vehicle (DMSO) or sulforaphane (SULF; 0.5 mg/kg per day, intraperitoneally) for 5 days and subjected to ITTs. (G to I) Six-month-old male Nox4fl/fl and Mck-Cre;Nox4fl/fl chow-fed male mice were fasted for 6 hours, and mice were subjected to hyperinsulinemic-euglycemic clamps; [14C]2-deoxy-d-glucose was administered at the end of the clamp. (G) The GIR, (H) EGP and RD were assessed under basal and clamped conditions. (I) Tissues were extracted, and [14C]2-deoxy-d-glucose uptake (Rg′) was assessed. Representative and quantified results are shown (means ± SEM) for the indicated number of mice; significance determined using (E, F, and H) two-way ANOVA, (E) one-way ANOVA, or (A, C, D, G, and I) a Student’s t test. In (E), * indicates significance for Nox4fl/fl versus Mck-Cre;Nox4fl/fl mice, and $ indicates significance for Nox4fl/fl versus Mck-Cre;Nox4fl/fl;Gpx1−/− mice. In (F), * indicates significance for Nox4fl/fl versus Mck-Cre;Nox4fl/fl mice, and $ indicates significance for Mck-Cre;Nox4fl/fl versus Mck-Cre;Nox4fl/fl + SULF mice.