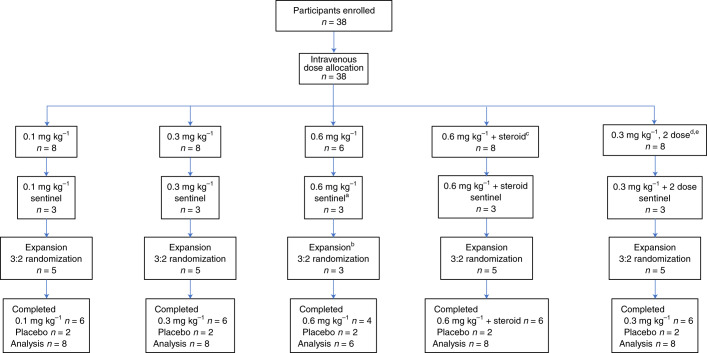
Fig. 1. Trial flow.
The study protocol planned to enroll 8 participants per treatment group. Six of 8 participants were allocated in the 0.6 mg kg−1 dose group due to the decision to augment the premedication regimen with steroid to assess its potential mitigation of infusion-related reactions (0.6 mg kg−1 + steroid group). aParticipants received loratadine and ranitidine 90 min before infusion. bParticipants received loratadine, ranitidine (sentinel, expansion) and acetaminophen (expansion) 90 min before infusion. cParticipants received steroid (dexamethasone) and diphenhydramine and famotidine 90 min before infusion. dParticipants received diphenhydramine and famotidine 90 min before infusion. eParticipants were administered two 0.3 mg kg−1 doses on days 1 and 8.