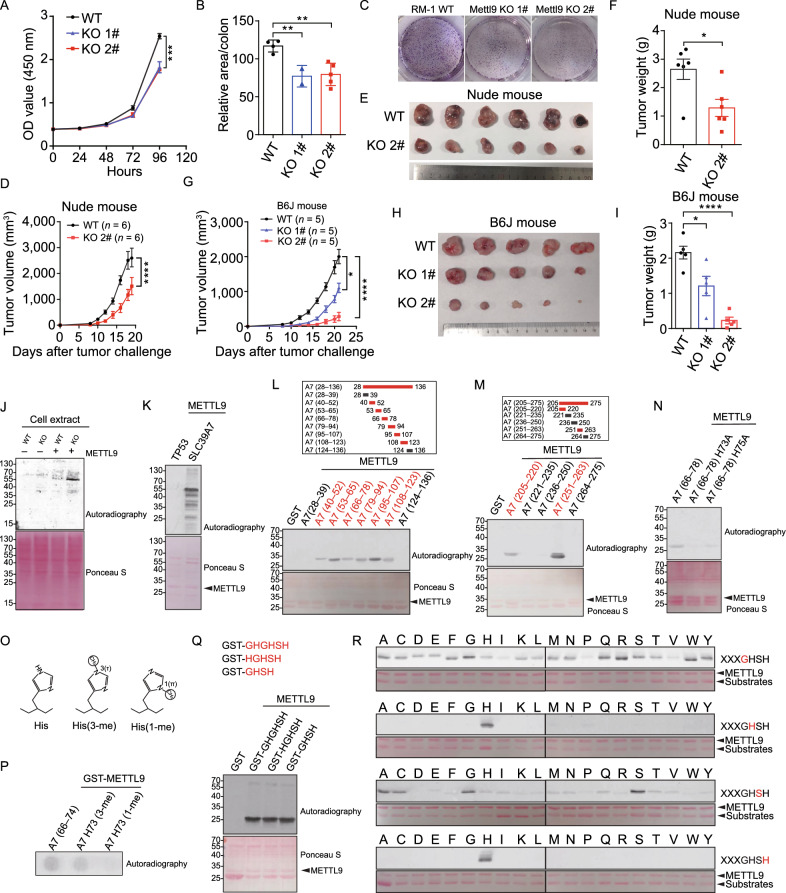
Figure 1.
METTL9 is a protein N1-histidine methyltransferase that is required for cell proliferation and tumor growth. (A–C) Knockout of Mettl9 in RM-1 tumor cells decreases cell growth. (A) Cell growth of wildtype (WT) and two different knockout (KO) clones of RM-1 cells was measured by CCK8 and (B and C) colony formation assay. (D–I) WT and Mettl9 KO RM-1 tumor cells were injected into nude mouse and C57B6/J mice. (D and G) Tumor growth curves in nude mice (n = 6) and C57B6/J mice (n = 5). (E and H) Pictures of tumors three weeks after tumor cell injection. (F and I) Tumor weights for (E and H). (J–N) Enzymatically active METTL9 methylates SLC39A7. (J) Fluorography showing the activity of recombinant METTL9 on cell extracts from WT and Mettl9 KO RM-1 cells in the presence of [3H]AdoMet. Ponceau S staining for total protein was used as loading control (bottom). (K) Fluorography showing recombinant GST-SLC39A7 was methylated by recombinant METTL9 in the presence of [3H]AdoMet. GST-P53 was used as a substrate control. (L and M) Fine mapping of the METTL9-methylated regions in SLC39A7. The methylated truncate was colored in red. (N) Fluorography showing in vitro activity of METTL9 on WT and mutated recombinant GST-A7 (66–78). (O) Histidine (His, left), 3-(τ)-methyl histidine (His(3-me), center); 1-(π)-methyl histidine (His(1-me), right). (P) METTL9 generates His73(1-me). In vitro methylation reactions with GST-METTL9 on the indicated peptides visualized by autoradiography. (Q) Fluorography showing GHSH motif is adequate for METTL9 mediated methylation. In vitro methylation assay of METTL9 on recombinant GST-GHGHSH, GST-HGHSH and GST-GHSH. (R) In vitro activity of METTL9 on recombinant protein arrays. Residue in the GHSH motif (red) was replaced with 20 different amino acids. For all panels, *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Error bars represent S.D. Data are representative of three independent experiments