Figure 2.
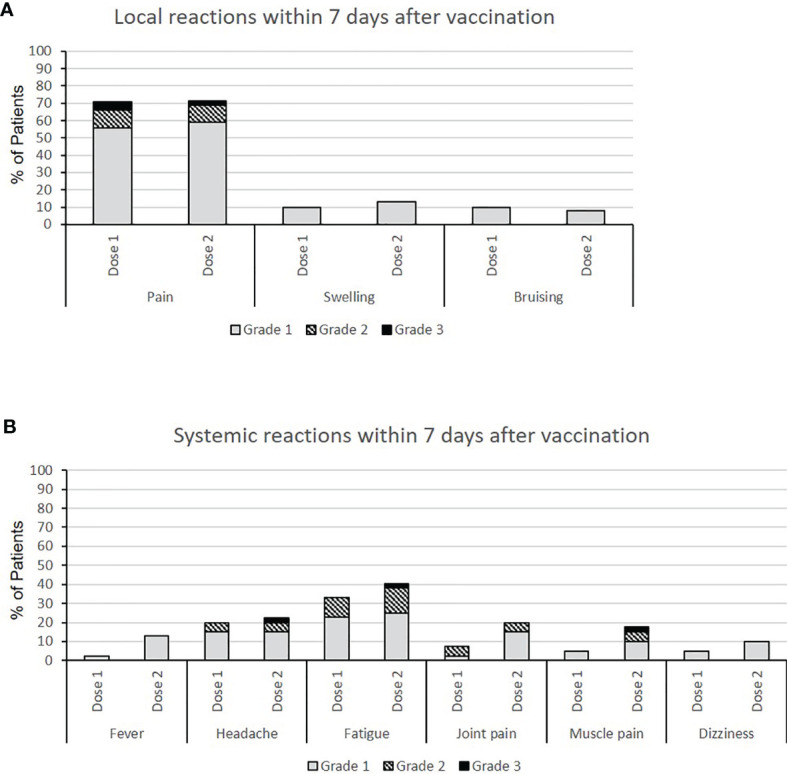
(A) Local and (B) systemic adverse events (AEs) after the first and the second dose of mRNA-1273 vaccine in 39 PD patients. The AEs were recorded using a standardized survey and the patients were asked to grade them using a scale from 0 to 4 (0=no event; grade 1=mild, does not affect daily activities; grade 2=moderate, interferes with activities of daily living; grade 3=severe, interrupts usual activities of daily living; grade 4=hospitalization).
