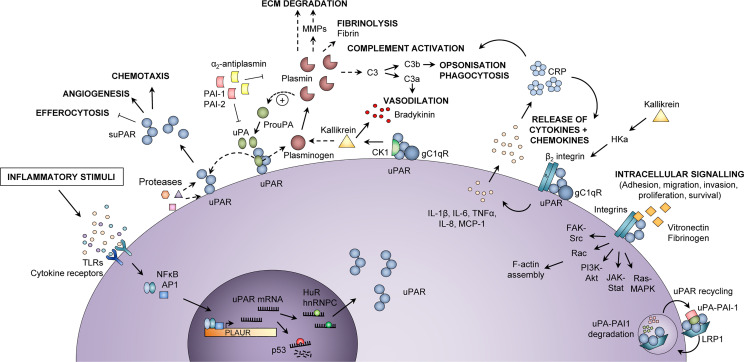
Figure 1.
Inflammatory functions of uPAR and suPAR. Upon an inflammatory stimulus, e.g., stimulation of toll-like receptors (TLRs) or cytokine receptors, the expression of urokinase plasminogen activator receptor (uPAR) in immunologically active cells is increased via activation of transcription factors, such as nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and activator protein 1 (AP1), which bind to the promoter region of the PLAUR gene. The uPAR mRNA is either degraded (by p53) or stabilized for translation (by HuR or hnRNPC), after which uPAR is expressed at the cell surface, bound to the membrane via a glycosyl phosphatidylinositol (GPI) anchor. At the cell surface, uPAR can become cleaved by various proteases or its own ligand urokinase plasminogen activator (uPA), thus generating suPAR, which plays a role in inflammation by impairing neutrophil efferocytosis and stimulating angiogenesis and chemotaxis. Active uPA cleaves plasminogen to plasmin, which in turn cleaves and activates uPA. Plasmin activates matrix metalloproteases (MMPs), cleaves extracellular matrix (ECM) components, degrades fibrin, and activates the classical complement pathway, thereby promoting migration and invasion of cells, fibrinolysis, vasodilation, opsonization, and phagocytosis of foreign pathogens. Co-localization of uPAR with the proteins cytokeratin-1 (CK1) and globular C1q receptor (gC1qR) on the surface of endothelial cells also promotes vasodilation through release of bradykinin via activation of kallikrein. In a complex with β2 integrin and gC1qR, uPAR also induces release of cytokines (IL-1β, IL-6, TNFα) and chemokines (IL-8, MCP-1), upon stimulation by cleaved high molecular weight kininogen (HKa). Cytokines stimulate the production of C-reactive protein (CRP) from the liver, and CRP itself functions as an opsonin and also activates the classical complement pathway. Furthermore, uPAR interacts with vitronectin, fibrinogen, and integrins, mainly αMβ2 integrin (Mac-1) but also β1 and β3 integrin complexes, activating intracellular signaling pathways that facilitate cell adhesion, migration, invasion, proliferation, and survival by affecting F-actin assembly and gene transcription. The activity of uPA and plasmin is inhibited by plasminogen activator inhibitor (PAI)-1, PAI-2, and α2-antiplasmin. Binding of PAI-1 and low-density lipoprotein receptor-related protein 1 (LRP1) mediates endocytosis of uPAR-uPA-PAI-1 complexes, followed by lysosomal degradation of uPA and PAI-1 and recycling of uPAR back to the membrane. In endothelial cells, co-localization of uPAR with CK1 and gC1qR activates kallikrein and promotes the release of the vasodilator bradykinin. hnRNPC, heterogeneous nuclear ribonucleoprotein C; HuR, Hu antigen R; IL, interleukin; MCP-1, monocyte chemoattractant protein-1; TNFα, tumor necrosis factor α. Adapted from Rasmussen, LJH (2018) (19) with permission.