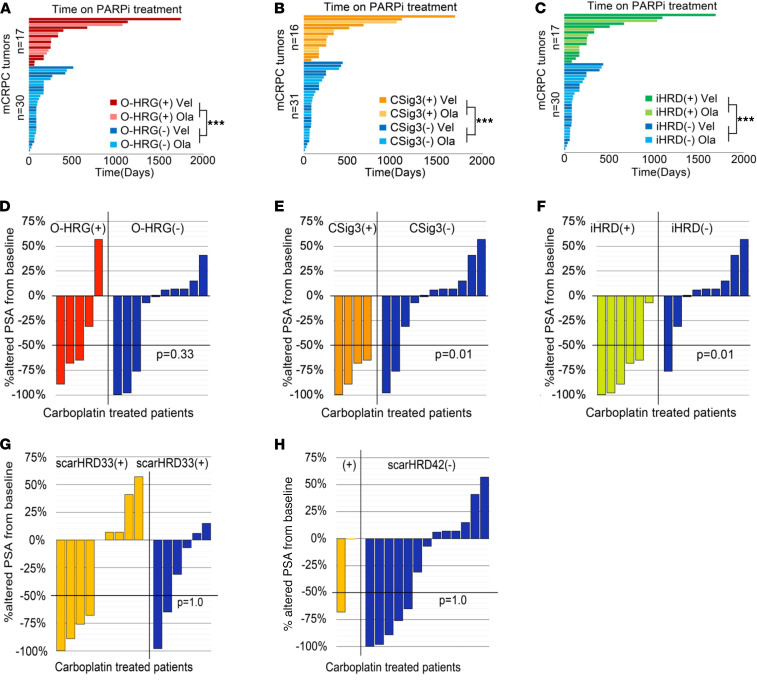
Figure 7. Clinical treatment outcomes associate with tumor COSMIC signature 3 and integrated assessment of HRR deficiency classification.
(A–C) Clinical outcomes of PARPi treatment shown by time (days) on drug depicted in swimmer plots partitioned by: (A) HRG mutation status: tumors are classified as O-HRG(+) or O-HRG(–) based on the biallelic loss of 1 of 14 genes approved for olaparib treatment; (B) CSig3 classification; (C) iHRD classification. (D–H) Clinical outcomes of carboplatin chemotherapy shown by the maximum decline in serum prostate-specific antigen measurements. Tumors are classified by (D) O-HRG(+) or O-HRG(–) based on biallelic loss of 1 of 14 genes approved for olaparib treatment; (E) CSig3 classification; (F) iHRD classification; (G) scarHRD classification using a score ≥33 as positive for HRRd; (H) scarHRD classification using a score ≥42 as positive for HRRd. Comparison of time on PARPi treatment was performed using Mann-Whitney U test, and associations of biomarker status to carboplatin PSA50 response was determined using Fisher’s exact test (P value *** < 0.001).