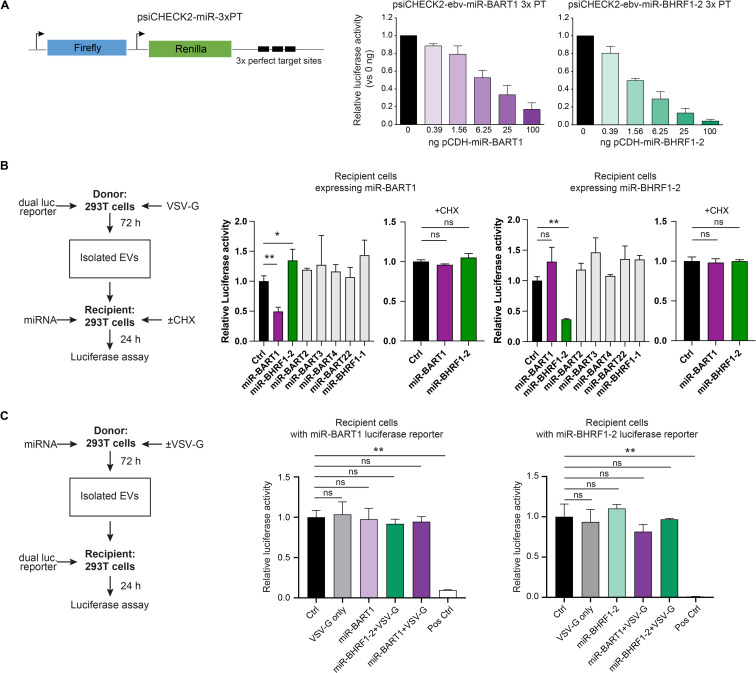
Fig 6. Dual luciferase reporter assays indicate a functional EV-mediated transfer of mRNA transcripts but fail to detect miRNAs-dependent regulation of reporter transcripts in recipient cells.
(A) The design of the modified dual luciferase reporter plasmid, based on psiCHECK2, is shown, which encompasses the internal control firefly luciferase (used for normalization) and the reporter Renilla luciferase with three tandem copies of perfect complementary target sites (3xPT) of the miRNAs of interest inserted in the 3′UTR of the Renilla mRNA. 293T cells were transfected with 30 ng of the miRNA reporter plasmid containing 3xPT with increasing amounts of the corresponding miRNA expression vector (pCDH) starting with 390 pg up to 100 ng. At 24 h after transfection cells were lysed to determine the Renilla and firefly luciferase activities. Mean of three replicates is shown. (B) Reciprocal dual luciferase assays with EVs engineered to transfer luciferase-encoding mRNAs to recipient 293T cells expressing viral miRNAs. Left panel: overview of the principal components of the dual luciferase assay. 293T donor cells seeded in a 13-cm dish were transiently transfected with 12 μg of 3x PT psiCHECK2 dual luciferase reporter plasmid DNAs (shown in panel A) together with 8 μg of a VSV-G expression plasmid. 50 μl of purified EVs (3.1x104 particles/cell) were transferred to recipient 293T cells in 24-well plates transiently transfected with 500 ng expression plasmid DNA encoding miR-BHRF1-2 or miR-BART1 as indicated. As control, recipient 293T cells were also transfected with 500 ng of expression plasmid DNAs encoding miR-BART2, miR-BART3, miR-BART4, miR-BART22 or miR-BHRF1-1. As another control, the recipient cells were incubated with cycloheximide (CHX; 20 μg/ml) to abrogate translation. Middle and right panels: results of reporter assays lysates from recipient 293T cells expressing miR-BHRF1-2 or miR-BART1 as indicated. The transduced mRNAs encoding Renilla luciferase with the perfect complementary target sites (3xPT) for miR-BHRF1-2 or miR-BART1 (and firefly luciferase used for normalization) are translated and expressed in the recipient cells, but repressed in cells that contain the matching miRNA. Mean and SD of three independent donors are shown. Asterisks indicate statistical significance by paired two-tailed t test. (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001). (C) Dual luciferase assays with reporter constructs (3x PT psiCHECK2) shown in panel A were performed to investigate the functional transfer of EV-borne viral miRNAs to 293T recipient cells. Left panel: overview of the dual luciferase assay. 293T cells seeded in a 13-cm dish were transiently transfected with expression plasmids (12 μg) coding for miR-BHRF1-2 or miR-BART1 alone or in combination with an expression plasmid coding for VSV-G (8 μg). Medium was replaced with fresh medium after 24 hours and the cells were incubated for another 72 h prior to harvest. 50 μl of EVs were transferred to 293T cells in a 24-wells plate transiently transfected with 10 ng of dual reporter 3x PT psiCHECK2 plasmid DNA as indicated. Middle and right panels: results of reporter assays show no repression of the luciferase reporters. Ctrl: conditioned medium from 293T donor cells transiently transfected with an expression plasmid encoding no miRNA; VSV-G: conditioned medium from 293T donor cells transiently transfected with a VSV-G encoding expression plasmid, only; Pos Ctrl: recipient 293T cells transiently co-transfected with both the miRNA expression plasmid and the corresponding 3x PT psiCHECK2 reporter plasmid. Mean and SD of three independent donors are shown. Asterisks indicate statistical significance by paired two-tailed t test. (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001).