Abstract
Research dealing with early diagnosis and efficient treatment in colon cancer to improve patient's survival is still under investigation. Chemotherapeutic agent result in high systemic toxicity due to their non‐specific actions on DNA repair and/or cell replication. Traditional medicine such as Lycopodium clavatum (LC) has been claimed to have therapeutic potentials against cancer. The present study focuses on targeted drug delivery of cationic liposomal nanoformulated LC (CL‐LC) in colon cancer cells (HCT15) and comparing the efficacy with an anti‐colon cancer drug, 7‐ethyl‐10‐hydroxy‐camptothecin (SN38) along with its nanoformulated form (CL‐SN38). The colloidal suspension of LC was made using thin film hydration method. The drugs were characterised using ultraviolet, dynamic light scattering, scanning electron microscopy, energy, dispersive X‐ray spectroscopy. In vitro drug release showed kinetics of 49 and 89% of SN38 and LC, whereas CL‐SN38 and CL‐LC showed 73 and 74% of sustained drug release, respectively. Studies on morphological changes, cell viability, cytotoxicity, apoptosis, cancer‐associated gene expression analysis of Bcl‐2, Bax, p53 by real‐time polymerase chain reaction and western blot analysis of Bad and p53 protein were performed. Nanoformulated LC significantly inhibited growth and increased the apoptosis of colon cancer cells indicating its potential anti‐cancer activity against colon cancer cells.
Inspec keywords: cancer, biological organs, cellular biophysics, drug delivery systems, drugs, nanomedicine, genetics, DNA, molecular biophysics, biochemistry, lipid bilayers, toxicology, suspensions, colloids, light scattering, X‐ray chemical analysis, solvation, enzymes, nanostructured materials
Other keywords: energy dispersive X‐ray spectroscopy, in vitro drug release, morphological changes, cell viability, cytotoxicity, apoptosis, cancer‐associated gene expression analysis, Bcl‐2, Bax, real‐time polymerase chain reaction, western blot analysis, Bad protein, p53 protein, scanning electron microscopy, dynamic light scattering, ultraviolet scattering, thin film hydration method, colloidal suspension, nanoformulated form CL‐SN38, 7‐ethyl‐10‐hydroxy‐camptothecin, anticolon cancer drug, colon cancer cells HCT15, cationic liposomal nanoformulated LC, targeted drug delivery, therapeutic potentials, Lycopodium clavatum, traditional medicines, cell replication, DNA repair, nonspecific actions, high systemic toxicity, chemotherapeutic agents, patient survival, colon cancer treatment, colon cancer diagnosis, CL‐LC, potential anticancer activity
1 Introduction
In recent years, the incidence of colon cancers is increasing steadily [1]. Alterations at the gene level due to genetic mutations or various environmental factors could lead to dysplasia of epithelial cells, which could progress to colon neoplasia through a complex mechanism [2]. Colon cancer is one of the most frequent cancers in human beings that has been characterised for the genetic alterations with tumour progression. It was the first tumour type, for which a model of carcinogenesis was proposed [3]. There is an increasing evidence showing that a rare population of undifferentiated cells are responsible for tumour formation and maintenance [4, 5]. Cancerous tissue, presents multiple barriers to the successful delivery of oxygen, nutrients and therapeutics, which are the ‘hallmarks’ of the microenvironment of solid tumours and include: (i) irregular and disorganised vasculature, (ii) high interstitial fluid pressure, (iii) low‐oxygen tension and hypoxia and (iv) low extracellular pH and a dense extracellular matrix [6, 7]. Although long‐standing efforts on early diagnosis and efficient treatment have been made to improve patient survival, the successes have not been subsequently confirmed and the benefits of chemotherapy are still under investigation. Attempts are, therefore, being made to solve this problem of delivering the drug to the target by reducing the toxic effects on normal tissue by developing nanoformulations for patients who are resistant to chemotherapy. Toxicity and side effects, necessitates the search for relatively non‐toxic drugs or natural products, to wage a more human war against cancer [8].
However, the drug should be more efficient against the cancer cells rather than the normal cells, by increasing the uptake and the bioavailability of the drug to the targeted cancer cells. Therefore, to be successful as a drug delivery system, nanoparticles must be able to target tumours which are localised outside mononuclear phagocyte system rich organs [9]. Chemotherapeutic agents result in high systemic toxicity due to their non‐specific actions on DNA repair and/or cell replication [10]. Anticancer treatments including ionising radiations, alkylating agents, hyperthermia, DNA topoisomerase inhibitors and platinum compounds that induce DNA damage indiscriminately, kill normal cells along with the rapidly proliferating cancer cells [11]. Even though it is toxic to normal cells, chemotherapy became a major therapeutic approach for the treatment of localised and metastasized cancers, where the disease is prevented by the administration of one or several natural drugs [12]. Non‐specificity of these drugs to cancer cells induce adverse side effects in patients, limiting the administrable dosage of drugs, which leads to incomplete tumour responses and hence cancer relapse [13].
Traditional plant‐based medicines still exert a great deal of importance to the people living from the ancient time and lead to discovery of new drug candidates for a variety of diseases that threaten human health [14, 15]. Lycopodium genus belongs to the family Lycopodiaceae [16]. Mandal et al. [17] demonstrated lycopodine as the main anticancer agent from Lycopodium clavatum (LC) extract. Lycopodine inhibited proliferation of HeLa cells through induced chromatin condensation, inter‐nucleosomal DNA fragmentation and enhanced cell population in sub‐G1 region along with an increase in reactive oxygen species generation, mitochondrial membrane potential depolarisation, with the release of cytochrome‐c and activation of caspase‐3, which are the events closely involved in apoptosis.
In the present paper, we aimed to investigate the effect of cationic liposomal nanoformulated LC (CL‐LC) in reducing growth of colon cancer cells.
2 Materials and methods
Methyl alcohol (MeOH), chloroform (CHCl3), cholesterol (CHOL), isopropanol, cetyl trimethylammonium bromide (CTAB), ethanol, sodium chloride (NaCl), 7‐ethyl‐10‐hydroxy‐camptothecin (SN38), LC spore powder, Dulbecco's modified eagle medium (DMEM), foetal bovine serum (FBS), trypsin and ethylene diamine tetra‐acetic acid were purchased. Tissue culture plastic wares, caspase‐cleaved cytokeratin‐18 (M‐30) apoptosis kit, lactate dehydrogenase (LDH) assay kit, 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide, S‐diphenyltetrazolium. Bromide (MTT) kit dimethyl sulphoxide along with all other chemicals used, were purchased.
2.1 Phospholipids isolation from egg yolk
Phospholipids were isolated from chicken egg yolk by using laboratory standard method [18]. To the 1 ml of egg yolk, 1 M NaCl was added to three times of its volume followed by vigorous vortex. About 12 ml of MeOH:CHCl3 (2:1) was then added and mixed well. To the contents, 1 ml of 1 M NaCl was added and mixed vigorously along with 3 ml of CHCl3, followed by centrifugation at 2000 rpm for 5 min. Separations of phases were observed and the organic phase containing phospholipid at the bottom was isolated carefully.
2.2 Preparation of CL by thin film hydration method
About 5 mg of CHOL was added to the 500 µl of the isolated phospholipids, mixed in 10 ml of CHCl3 :MeOH (3:1) along with 300 µl of CTAB in a round bottom flask which was allowed to dry using rotary‐evaporator connected with a vacuum pump. The thin film formed on the sides of the flask was kept in vacuum desiccators overnight so that it dries completely. About 20 ml of phosphate‐buffered saline (PBS) was then added to it and sonicated for 60 min at room temperature at a frequency of 40 kHz to facilitate the formation of liposomal binding. The formulated liposome was characterised by microscopic observation, ultraviolet–visible (UV–vis) spectroscopy and dynamic light scattering (DLS).
2.3 Preparation of CL‐LC and CL‐SN38
The drug; SN38 was loaded to CL samples to make 125 µM as stock and was sonicated for 60 min at room temperature. The nanoformulated drug was characterised by using UV–vis spectroscopy, DLS and scanning electron microscopy (SEM)‐EDAX.
The Lycopodium clavatum spore powder is usually water insoluble. So, the nano‐formulation of LC was performed through a colloidal suspension followed by the thin film hydration method. The Lycopodium clavatum was directly added to the RB flask along with 5 mg CHOL, 500 µl phospholipids and 10 ml of CHCl3 : MeOH and 300 µl of CTAB were subjected to the formation of liposomal encapsulated thin film and it was allowed to dry using a rotary – evaporator connected to a vacuum pump. The thin film that was formed was allowed to dry out by keeping the flask in a vacuum desiccator, overnight. About 20 ml of PBS was then added to it and sonicated for 60 min at room temperature at a frequency of 40 kHz to facilitate the formation of liposomal encapsulation of 2 mM as a stock drug. The nanoformulated drug was characterised by using UV–vis spectroscopy, DLS and SEM‐EDAX.
The drug loaded liposomal samples were then centrifuged at 20,000 rpm for 45 min and the optical density of the supernatant and pellet were noted at 380 nm for SN38‐loaded liposomes and at 260 nm for the colloidal suspended liposomal LC to calculate their drug loading efficacy.
2.4 In vitro drug release kinetics
Drugs loaded to CL were sealed with a porous dialysis membrane and the drugs were allowed to release in a 25 ml tube containing PBS by creating an in vitro circulation at pH 7.4 at room temperature. The 2 ml of PBS containing the released nanoformulated drugs was withdrawn at 15 min time intervals in exchange for adding fresh 2 ml PBS, to measure the OD at the λ max of the drug for calculating the drug release. Finally, the percentage of drug release was calculated.
2.5 Cell lines and cell culture conditions
Colon cancer cell line (HCT15) was procured from the National Cancer for Cell Science, Pune. The cell lines were grown in DMEM supplemented with 10% FBS and 1% antibiotic and incubated at 37°C incubator with 5% carbon dioxide (CO2). Cells were harvested carefully with 0.025% trypsin and plated at required cell numbers and allowed to adhere for 24 h before treatment.
2.5.1 In vitro cell viability assay
Cells were aspirated and diluted using sterile PBS at (pH 7.2). To the cell suspension, variable concentrations of the test drugs were added and these assay mixtures were incubated for 24 h at 37°C in a CO2 incubator. To determine the effects of nanoformulated LC and SN38 in HCT15 cells, cell viability test was performed using MTT assay (19). In a 96 well plate, 5000 cells per well were seeded and incubated for 24 h. Cells were exposed to nanoformulated LC and SN38 at various concentrations (7, 10, 13, 25, 64 µM for CL‐SN38 and 12, 16, 20, 40, 100 µM for CL‐LC) and incubated for 48 h. The intracellular formazan crystals formed were solubilised with DMSO and the absorbance of the solution was measured at a wavelength of 570 nm. The IC50 value was determined.
2.6 Assessment of cell morphology
HCT15 cells were plated in six‐well culture plates (5000 cells/well) in DMEM cells supplemented with 10% FBS for 48 h and treated with liposomal SN38 and liposomal LC at a different concentration and observed under inverted phase contrast microscope after 48 h.
2.7 LDH assay
The cells treated with nanoformulated drugs were allowed to incubate for 24 h in CO2 incubator and after the incubation the media were collected as lysis solution. In a 96‐well plate, 10 µl of lysis solution was added to LDH control wells, 10 µl test compounds to the experimental wells, 10 µl PBS to untreated control wells and 10 µl solvent to the vehicle wells. The plate was incubated in a CO2 incubator at room temperature for 40–45 min. About 50 µl of supernatant from each well were taken in another 96‐well plate, and to each well the LDH reagent was added followed by 20–30 min of incubation at room temperature. Finally, absorbance was measured at 490 nm which was used as a main wavelength and 600 nm as a reference wavelength.
2.8 M‐30 apoptosis assay
Apoptosis was quantitatively detected using M‐30 apoptosis enzyme‐linked immunosorbent assay (ELISA) kit (Sweden). The M30 apoptosis ELISA is a solid‐phase sandwich enzyme immune‐assay that measures the level of soluble caspase cleaved CK‐18, containing the M‐30 neo‐epitope. This assay is based on M‐30 neo‐epitope monoclonal antibody, which was exposed in HCT15 cells as the epithelial cell specific marker CK‐18 cleaved by caspases during early apoptosis. Control, standard and samples react with the solid capture M‐30 antibody directed against M‐30 neo‐epitope. Unbound conjugates were removed and horse radish peroxidase substrate was added. The developing colour shows the increasing concentration.
2.9 RNA isolation
RNA was extracted from the analysed material, using the phenol–CHCl3 method by the total RNA Prep Plus. All extracts were treated with DNAse I to avoid contamination of genomic DNA. The RNA was quantitated spectrophotometrically.
2.9.1 cDNA synthesis and real‐time polymerase chain reaction
cDNA was synthesised using random primers and MuLV reverse transcriptase (Applied Biosystems, Foster City, CA, USA). Quantitative polymerase chain reaction (qPCR) was performed with SYBR green qPCR Master Mix (Applied Biosystems), primers designed in two adjacent exons using Primer Express® software with an ABI PRISM 7900 sequence detection system. All data were normalised to glyceraldehyde 3‐phosphate dehydrogenase expression. Oligonucleotide primers used for qRT‐PCR are listed in Table 1.
Table 1.
Listed oligonucleotide primers used for qRT‐PCR
| Gene | Sequence (5′<‐‐sequence‐‐>3′) |
|---|---|
| β ‐actin f | GCTGATCCACATCTGCTGG |
| β ‐actin r | ATCATTGCTCCTCCTCAGCG‐ |
| Bcl‐2: f | CGCCCTGTCGATGACTGAGTA |
| Bcl‐2: r | CCCATGCTCCGTTATCCTG |
| Bax: f | TGG AGCTGCAGAGGATGATTG |
| Bax: r | GAAGTTGCCGTCAGAAAACATG |
| p53:f | AACGGTACTCCGCCACC |
| p53:r | CGTGTCACCGTCGTGGA |
2.10 Western blot analysis of Bad and p53 protein expression
In brief, aliquots of protein extracts (30 µg) from cells of different treatment groups were suspended in 0.1 M Tris‐HCl buffer (pH 7.4) containing 1% sodium dodecyl sulphate (SDS), 0.05% β ‐mercaptoethanol, 2.5% glycerol and 0.001% bromophenol blue, followed by fractionation by 10% SDS polyacrylamide gel electrophoresis. Proteins were transferred electrophoretically onto nitrocellulose membranes (0.2 µM). Membranes were blocked using 5% non‐fat dry milk and 0.1% Tween 20 in Tris‐BS (TBS) and subsequently probed with primary antibody of Bad or p53 in TBS containing 3% non‐fat dry milk and 0.1% Tween 20. Antibody–antigen complexes were detected using goat anti‐rabbit IgG peroxidase conjugates following detection using an enhanced chemiluminescence kit (Amersham Corp).
Statistical analysis : One‐way student's t ‐test was used to determine statistical significance, p < 0.05 was considered statistically significant.
3 Results and discussion
3.1 Liposomes formation
Liposomes were prepared by thin‐layer hydration method using optimised concentration of CHOL (5 mg) and extracted PL (500 µl). Liposomal morphology and shape were monitored by optical microscopy (Fig. 1 a). Spherical liposomes were accurately observed under the oil immersion, 100 × dimension of the microscope.
Fig. 1.
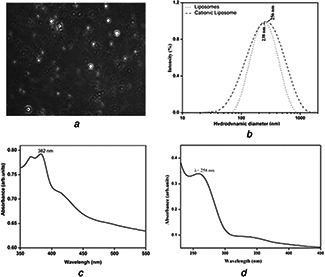
Liposomal morphology and shape were monitored by optical microscopy
(a) Microscopic image of liposome, (b) DLS spectrums of liposome and CL (cationic liposome), (c) UV–vis‐spectrums of CL‐SN38, (d) UV–vis‐spectrums of CL‐LC
The hydrodynamic diameter of the prepared liposome was determined by using DLS method at 25°C. The major scattering peak obtained at 242 nm (256 nm) (Fig. 1 b). The shift of cationic charge was observed from −88 to +242 eV for the cationic CTAB on the liposomal surface, which plays an important role for the targeted drug delivery in certain cases.
3.2 Nanoformulation of SN38 and LC
Liposomal encapsulation to the drugs was confirmed by a spectrophotometric study. The UV spectroscopic spectrum at λ max 380 nm (Fig. 1 c) shows the absorbance of cationic liposome encapsulated SN38 at λ max 382 nm, whereas the spectrum at λ max 270 nm (Fig. 1 d) shows the absorbance of colloidal suspended LC at 258 nm.
The sizes of the nanoformulated particles were determined by obtaining DLS spectra at a temperature of 25°C. The major scattering peak (Figs. 2 a and b) at 221 and 154 nm shows the average hydrodynamic diameter of liposomal SN38 and LC, respectively.
Fig. 2.
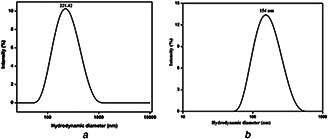
Major scattering peak
(a) Hydrodynamic graph of CL‐SN38, (b) Hydrodynamic graph of CL‐LC
The synthesised liposomal SN38 and liposomal LC were subjected to SEM analysis to determine the topography that showed successful integration of cationic liposomes onto the drugs. The observed microscopic image of liposomal SN38 (Fig. 3 a) and liposomal LC (Fig. 3 b) sample is shown at different magnifications. The elemental composition of the particles was determined using EDAX (Figs. 4 a and b). The cumulative drug release of both CL‐SN38 and CL‐LC were shown in Fig. 5.
Fig. 3.
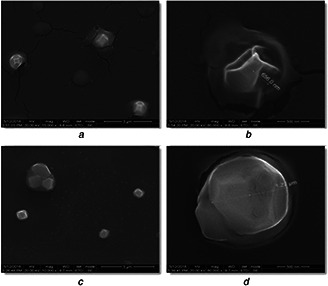
Observed microscopic image of liposomal SN38 and liposomal LC sample
(a) SEM image of a group of CL‐SN38 and a close‐up SEM image of CL‐SN38 prior to functionalisation, (b) SEM image of a group of CL‐LC and a close‐up SEM image of CL‐LC prior to functionalisation
Fig. 4.
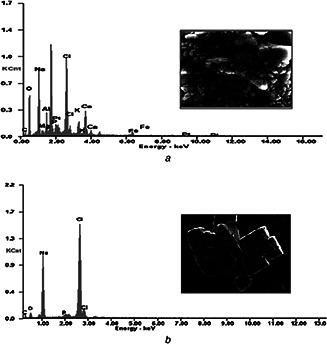
EDAX spectral analysis of
(a) CL‐SN38 liposomal LC, (b) Its elemental compositions
Fig. 5.
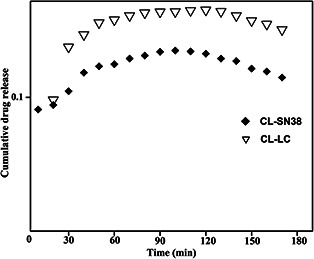
Cumulative drug release of both CL‐SN38 and CL‐LC
3.3 Assessment of cell morphology
Colon cancer cells (HCT15) plated in six‐well culture plates (5000 cells/well) in DMEM supplemented with 10% FBS for 48 h and treated with liposomal SN38 and liposomal LC at a desired concentration were observed with the help of inverted phase contrast microscope after 48 h and after the treatment with liposomal SN38 and liposomal LC, cancer cell's morphology was changed and cells became round in shape (Figs. 6 a and b).
Fig. 6.
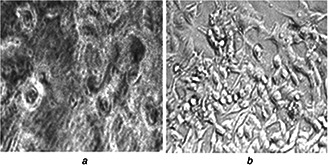
Cell morphology assessment of colon cancer cells HCT15 observed under inverted microscope
(a) Liposomal SN38, (b) Liposomal LC
3.4 Cytotoxicity effect of CL‐SN38 and CL‐LC on HCT15 cells
Cell viability of the colon cancer cells was performed by MTT assay. Fig. 7 showed the colon cancer cell viability calculated after the treatment with liposomal SN38 (Fig. 7 a) and liposomal LC (Fig. 7 a) in different concentrations. For CL‐SN38 IC50 value 2.3 µM and for CL‐LC, it was 130 µM and further morphological changes were observed (Figs. 6 a and b).
Fig. 7.
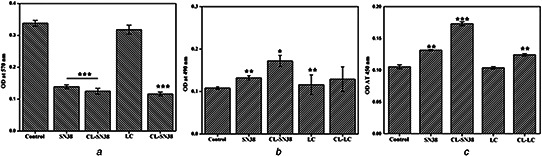
Colon cancer cell viability
(a) MTT assay of HCT15 colon cancer cells, (b) LDH assay of HCT15 colon cancer cells, (c) M‐30 apoptosis assay of HCT15 colon cancer cells
3.5 LDH assay
LDH assay was performed on the liposomal SN38 and liposomal LC treated colon cancer cells generating LDH, which were finally determined by taking the absorbance. At 490 nm as main wavelength and 600 nm as reference wavelength, Fig. 7 b represents increased OD, meaning increased LDH release, which is a prominent marker of cytotoxicity. Liposomal SN38 and liposomal LC showed greater response as compared with SN38 and LC.
3.6 Apoptosis assay
Fig. 7 c represents M‐30 apoptosis assay for liposomal SN38, liposomal LC, SN38 and LC on HCT15 cell lines. DMSO‐treated cells were taken as control because SN38 and LC were dissolved in DMSO (vehicle). Liposomal SN38 and CL‐LC showed increased apoptosis as compared with SN38 and LC. M‐30 apoptosis assay is related to caspase‐3 pathway. So probably the liposomal SN38 and liposomal LC works through caspase‐3 pathway to induce apoptosis in colon cancer cell (Fig. 7 c).
3.7 Real‐time PCR study of Bax and Bcl‐2 mRNA expression by SN38, CL‐SN38, LC and CL–LC
The effects of CL‐SN38, CL‐LC, CL‐SN38 and CL‐LC particles on the mRNA expression of Bax and Bcl2 were studied by real‐time PCR. Fig. 8 a represented Bax and Bcl2 data (Fig. 8 b) and it showed that CL‐SN38 and CL‐LC exert their effect, independent of Bax/Bcl2 pathway because they did not induce any changes in Bax/Bcl2 ratio in both the treatments, whereas SN38 and LC possibly works through Bax/Bcl2 pathway.
Fig. 8.
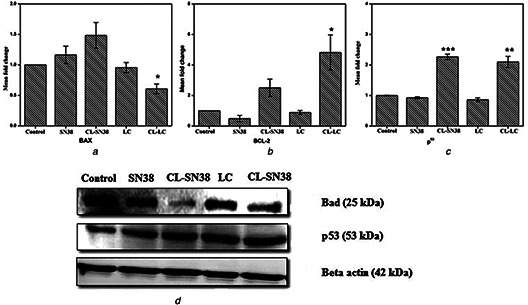
Showing the gene expression profiles of
(a) Bax, (b) Bcl‐2 and p53, (c) Western blot analyses of Bad and p53
3.8 Western blot analysis of Bad and p53 expression
Western blot analysis (Fig. 8 c) revealed that the pro‐apoptotic factor BAD related to Bcl2/Bax pathways remain unaffected by the treatment of liposomal SN38 and liposomal LC. Increasingly, that level of p53 was increased. So the present study suggest liposomal SN38 and liposomal LC possess anti‐cancer activities. That nanoformulated drug induced apoptosis, through caspase‐3 pathway, whereas SN38 and LC work through Bax/Bcl2 pathway.
4 Conclusions
Various therapeutical importance of LC has been studied due to its medicinal properties. The present paper focuses on targeted drug delivery of colloidal suspended CL‐LC on HCT15 colon cancer cells and comparing the efficacy with a well known conventional anti‐cancer drug for colon, SN38 and its CL nanoformulation. From the above work, anti‐cancer effect of LC has been proven with the help of nanotechnology, which played an important role to tune its effect and morphology along with the surface charge. In vitro drug release kinetics study concluded 73 and 74% of sustained drug release of nanoformulated LC and SN38, respectively. Studies on apoptosis showed that it had significantly increased in nanoformulated drugs, which are closely involved in anti‐cancer activity. CL‐LC considerably inhibited growth of colon cancer cells, which indicates its potential use in colon cancer treatment. Gene expression studies suggest that nanoformulated Lycopodium and SN38 possibly work through caspase‐3 pathway because Bcl2/Bax pathway remain unaltered after the treatment of nanoformulated drugs in colon cancer cells.
In the present paper, the results of several widely accepted protocols, unequivocally suggests that LC has anti‐cancer potentials against colon cancer when it is nanoformulated using cationic liposome.
5 Acknowledgments
The authors are thankful to Chettinad Academy of Research and Education (CARE) and Swedish Research Council for providing the funding and Dr. Agnishwar Girigoswami for help in preparation of liposomal nanoparticles. This study was financially supported by grants from the Chettinad Academy of Research and Education, Chennai, India and Swedish Cancer Foundation, Swedish Research Council and the Health Research Council in the South–East of Sweden and grant from Swedish Research Council, Sweden.
6 References
- 1. Ilyas M. Straub J. Tomlinson I.P. et al.: ‘Genetic pathways in colorectal and other cancers’, Eur. J. Cancer, 1999, 14, pp. 1986 –2002 [DOI] [PubMed] [Google Scholar]
- 2. Pardal R. Clarke M.K. Morrison M.J.: ‘Applying the principles of stem‐cell biology to cancer’, Nat. Rev. Can., 2003, 3, pp. 895 –902 [DOI] [PubMed] [Google Scholar]
- 3. Fearon E.R. Vogelstein B.: ‘A genetic model for colorectal tumorigenesis’, Cell, 1990, 3, pp. 759 –767 [DOI] [PubMed] [Google Scholar]
- 4. Bonnet D. Dick J.E.: ‘Human acute myeloid leukemia is organize as a hierarchy that originates from a primitive hematopoietic cell’, Nat. Med., 1997, 3, pp. 730 –737 [DOI] [PubMed] [Google Scholar]
- 5. Singh S.K. Hawkins C. Clarke I.D. et al.: ‘Identification of human brain tumour initiating cells’, Nature, 2004, 7015, pp. 396 –401 [DOI] [PubMed] [Google Scholar]
- 6. Cairns R. Papandreou I. Denko N.: ‘Overcoming physiologic barriers to cancer treatment by molecularly targeting the tumor microenvironment’, Mol. Cancer Res., 2006, 4, pp. 61 –70 [DOI] [PubMed] [Google Scholar]
- 7. Waite C.L. Roth C.M.: ‘Nanoscale drug delivery systems for enhanced drug penetration into solid tumors: current progress and opportunities’, Crit. Rev. Biomed. Eng., 2012, 40, pp. 21 –41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pan M.‐H. Ho C.‐T.: ‘Chemopreventive effects of natural dietary compounds on cancer development’, Chem. Soc. Rev., 2008, 11, pp. 2558 –2574 [DOI] [PubMed] [Google Scholar]
- 9. Mohanraj V.J. Chen Y.: ‘Nanoparticles – a review’, Trop. J. Pharm. Res., 2005, 5, pp. 561 –573 [Google Scholar]
- 10. Kehrer D.F.S. Soepenberg O. Loos W.J. et al.: ‘Modulation of camptothecin analogs in the treatment of cancer: a review’, Anti‐cancer Drugs, 2001, 12, pp. 89 –105 [DOI] [PubMed] [Google Scholar]
- 11. Essack M. Bajic V.B. Archer J.A.: ‘Recently confirmed apoptosis‐inducing lead compounds isolated from marine sponge of potential relevance in cancer treatment’, Mar. Drugs, 2011, 9, pp. 1580 –1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bisht S. Maitra A.: ‘Dextran–doxorubicin/chitosan nanoparticles for solid tumor therapy’, Wiley Interdiscip. Rev., Nanomed. Nanobiotechnol., 2009, 14, pp. 415 –425 [DOI] [PubMed] [Google Scholar]
- 13. Chang H.R.: ‘Trastuzumab based neoadjuvant therapy in patients with HER2 positive breast cancer’, Cancer, 2010, 116, pp. 2856 –2867 [DOI] [PubMed] [Google Scholar]
- 14. Orhan I. Küpeli E. Şener B. et al.: ‘Appraisal of anti‐inflammatory potential of the club moss Lycopodium clavatum L.’, J. Ethnopharmacol., 2007, 109, pp. 146 –150 [DOI] [PubMed] [Google Scholar]
- 15. Corns C.M.: ‘Herbal remedies and clinical biochemistry’, Ann. Clin. Biochem., 2003, 40, pp. 489 –507 [DOI] [PubMed] [Google Scholar]
- 16. Lawrence G.H.M.: ‘Taxonomy of vascular plants’, vol. 1989 (MacMillan Publishing Co., New York, USA, 1951), pp. 337 –338 [Google Scholar]
- 17. Mandal S.K. Biswas R. Bhattacharyya S.S. et al.: ‘Lycopodine from Lycopodium clavatum extract inhibits proliferation of HeLa cells through induction of apoptosis via caspase‐3 activation’, Eur. J. Pharmacol., 2010, 626, pp. 115 –122 [DOI] [PubMed] [Google Scholar]
- 18. Jinachandran K. Gunasekaran A. Haseena Y. et al.: ‘Gene expression profile induced by liposomal nanoformulation of anticancer agents: insight into cell death mechanism’, Adv. Sci. Eng. Med., 2014, 6, (4), pp. 159 –165 [Google Scholar]


