Abstract
A biological method for synthesising silver nanoparticles (AgNPs) was developed using the callus extracts from Artemisia annua L. under sunlight at 25,000 lx. The AgNPs were characterised using transmission electron microscopy, atomic force microscope, X‐ray diffraction and Fourier transform infrared spectroscopy. The AgNPs were mostly spherical with the size of 2.1 to 45.2 nm (average 10.9 nm). Pulse treatments of AgNPs at 125, 250 and 500 mg/l for 1 h extended vase life of cut carnation (Dianthus caryophyllus cv. Green Land) flowers. Four dominant bacteria strains Arthrobacter arilaitensis, Kocuria sp., Staphylococcus equorum and Microbacterium oxydans were isolated from the stem‐ends of cut D. caryophyllus flowers. AgNP pulse inhibited significantly bacterial growth in vase solution and cut stem ends during all of the vase period. The bacteria related blockage in the stem‐ends was significantly alleviated by AgNP pulse because of its higher antibacterial efficacy against the dominant bacteria. In addition, ethylene release of cut carnation flowers was inhibited in response to AgNP pulse. This is the first time that the biologically synthesised AgNPs could be applied as a promising preservative agent for cut carnation flowers.
Inspec keywords: nanofabrication, silver, nanoparticles, microorganisms, transmission electron microscopy, atomic force microscopy, X‐ray diffraction, Fourier transform infrared spectra
Other keywords: biosynthesis, silver nanoparticles, Artemisia annua callus, stem end bacteria, cut carnation flowers, biological method, transmission electron microscopy, atomic force microscope, X‐ray diffraction, Fourier transform infrared spectroscopy, Dianthus caryophyllus cv. Green Land, Arthrobacter arilaitensis, Kocuria sp, Staphylococcus equorum, Microbacterium oxydans, ethylene release, time 1 h, Ag
1 Introduction
Synthesis of novel silver nanoparticles (AgNPs) with enhanced activity for medical and agricultural applications is a subject of constant interest [1]. Generally, AgNPs can be synthesised by chemical reduction, photochemical reduction, or by the physical methods, such as attrition and pyrolysis [2]. However, drawbacks of above methods include low yield, high expense, energy consumption in the physical approaches, and contamination in the wet‐chemical procedure. Hence, it is much expected to develop environmentally friendly processes for AgNP synthesis. Currently, synthesis of AgNPs using microorganism [3], enzyme [4] and plants or plant extracts [5, 6] is considered an ecofriendly alternative. Naturally, both the primary and secondary metabolites from plants such as polysaccharides, polyphenols, flavonoid glycosides and terpenoids, are powerful reducing agents or stabilisers for the production of AgNPs [7]. Hence, extracts rich in bioactive secondary metabolites from medicinal plants such as Ginko biloba [8], Aloe vera [9] and Morinda citrifolia [10], have been chosen to carry out the green synthesis of AgNPs with promising biological potentials.
Artemisia annua L. is a well‐known Chinese medicinal herb for the biosynthesis of antimalarial drug artemisinin [11]. Apart from artemisinin, several other bioactive terpenoids, flavonoids, coumarins, and steroids are also found in A. annua to possess antimicrobial, allelopathic, anti‐inflammatory, antifeedant and antitumour activities [12]. Recently, A. annua leaf extracts have been applied for green synthesis of AgNPs and the biosynthesised AgNPs have antibacterial, antioxidant and tyrosinase inhibitory activities [13, 14]. Interestingly, it was observed that some callus extracts were more efficient in the fabrication of AgNPs and the synthesised AgNPs were more distinct and scattered in distribution than those synthesised by intact leaf extract [15]. Plant callus can be suitable for large‐scale synthesis of AgNPs according to their rapid scale‐up in an in vitro culturing system. Callus from different plants such as Carica papaya L. [16], Catharanthus roseus var. alba [17], Sesuvium portulacastrum L. [15] and Medicago sativa L. [18] have been used successfully for AgNP synthesis.
Recently, nanotechnology has amplified the effectiveness of AgNPs as antimicrobial agents in medical application [19]. However, little research on the applicability of nanosilver has been reported for controlling agricultural diseases, particularly those caused by phytopathogenic bacteria [20, 21]. Living bacteria in the stem end of cut flowers are attributed largely to vascular occlusion and thereby reduce vase life of cut flowers [22]. Besides the blockage, stem‐end bacteria can also secrete extracellular virulence factors including enzymes, hormones or toxic compounds to accelerate senescence [23]. Vascular occlusions and associated wilting in cut carnation (Dianthus caryophyllus L.) usually occurred when the number of bacteria in vase water reached 107 –1011 colony forming units (CFU)/ml [24, 25]. Inclusion of chemical germicides such as silver thiosulphate, silver nitrate (AgNO3), and 8‐hydroxyquinoline citric (HQC) and plant extracts could prevent growth of bacteria in vase water and stems of cut carnation flowers [26, 27]. Recently, AgNPs with the properties of lower cost, broad‐spectrum activity have received attention to prolong the longevity of cut flowers. AgNPs were reported to alleviate effectively the bacteria‐related blockage in cut roses and extend their vase life [28, 29]. AgNP pulse could inhibit bacteria growth in the vase solution and in the stems of cut gerbera flowers [30]. Liu et al. [31] further evaluated the effects of different formulations of chemically synthesised AgNP and found that neutral and acidic AgNP pulse could be a potential preservative agent for extending the vase‐life of cut Acacia holosericea. Although there have been some reports on pulse or vase solution treatment of AgNPs to prolong the vase life of cut carnation flowers [32, 33, 34], little has been reported on AgNPs for controlling the bacteria‐related blockage to extend vase life in cut carnation. Moreover, there has not been any information on synthesis and properties of the applied AgNP therein. Therefore, as a follow‐up to our efforts on exploring AgNP for controlling phytopathogenic microbes [35] and biotechnological application of A. annua [36], we therefore wish to report a biological method to synthesise AgNPs using the callus extracts from A. annua. Transmission electron microscopy (TEM), atomic force microscope (AFM), X‐ray diffraction (XRD) and Fourier transform infrared spectroscopy (FTIR) were used to characterise AgNPs obtained from A. annua. To understand the role of AgNPs on extending vase life of carnation cut flowers, the effect of the biosynthesised AgNPs on vascular blockage and dominant bacterial strains isolated from the stem‐ends were investigated.
2 Materials and methods
2.1 Preparation of A. annua callus
The seeds of A. annua (cv. CQF39), obtained from Yunnan Academy of Agricultural Sciences in China, were sterilised with 0.1% (w/v) mercuric chloride for 60 s and germinated on MS medium [37] containing 2% (w/v) sucrose at pH 5.8. Germination started within 7 days and plantlets were used for callus induction. The young leaves of 3‐week old seedlings were cut to about 0.5 cm2 and induced for callus on MS medium containing 0.5 mg/l 6‐benzylaminopurine (6‐BA) and 1.0 mg/l 1‐naphthaleneacetic acid (NAA) in darkness at 25 ± 1°C. The calli were subcultured every 20 days.
2.2 Synthesis of AgNPs
In preparation of callus extracts, 20 g of fresh calli (20‐day culture) were ground to 100 mL sterile distilled water. The mixer was boiled (5 min), cooled and filtered using Whatman filter paper No. 1. The filtrate was stored at 4°C as the stock solution and was used within 1 week [16]. The stock solution of callus extract (5 ml) was added to 45 ml aqueous AgNO3 solution at 1.0 mM. The absorbance of the reaction mixture was monitored in the range of 300–600 nm using UV‐vis spectrophotometer (UV‐2600, Shimadzu Corporation, Japan) to find the absorbance peak [15].
To study the effect of sunlight intensities and time duration on the fabrication of AgNPs, 45ml AgNO3 at 1 mM was mixed with 5 ml of callus extract in a 150 ml Erlenmeyer flask and incubated in different sunlight intensities (4500, 6500, 10,000 and 25,000 lx). The absorbance of the reaction mixture was measured every 20 min. To remove excess silver ions, the silver colloidal solution was centrifuged at 22,000g for 30 min, washed three times with distilled water and dried in a freeze drier [38].
2.3 Characterisation of AgNPs
The size and morphology of the AgNPs were observed by TEM (H‐600, Hitachi, Japan) and AFM (AFM‐Solver Next, NT‐MDT, Russia). The XRD patterns were obtained using a Philips X'Pert Pro MPD (Cu‐Kα radiation) diffractometer. The freeze‐dried powder of synthesised AgNPs were ground with KBr pellets and subjected to FTIR analysis (Model FTS 7000, Varian Inc., USA).
2.4 AgNP pulse treatment
Cut carnation (D. caryophyllus cv. Green Land) flowers purchased from a local market were covered with plastic film and transported within 1 h to our laboratory. Flowering stems free of visual defects and with uniform size were used in the experiments. Prior to treatments, the stems were re‐cut to ∼25 cm length. All leaves were removed other than the upper two leaves. The flowers were kept in vase in a room at 23 ± 2°C under 12‐h photoperiod with 15 μmol/m2 s light intensity provided by cool‐white fluorescents tubes (Philips 55 W, the Netherlands) with a relative humidity of 60 ± 10%.
AgNP pulse treatments were carried out according to the method described by Lü et al. [29] with some modifications. Controls were pulsed with distilled water. Three concentrations (125, 250 and 500 mg/l) of AgNPs for pulse treatments for 1 h were used in experiments. The distilled water was immediately used in vases as the preservation solution after the pulse treatment. Vase life was determined from the time when the cut flowers were put into the preservation solution to the time when flower petals showed clear inward rolling and wilting [25]. The weight of vases with flowers was measured daily to determine the water loss. Average daily water loss was calculated as: water loss (g/stem d) = (Ct ‐1 –Ct ); where Ct is the weight at t =days 1, 2, 3, etc., and Ct ‐1 is the weight on the previous day [39]. The flower samples for ethylene production were taken at day 1, 3, 5, 7 and 9. Ethylene production was quantified by gas chromatography (GC9790II, Fuli Analytical Instrument, China) according to method described by Tanase et al. (2015) [40]
2.5 Bacterial counts
To determine bacterial populations in vase solution, aliquots (0.1 ml) from three replication vases were taken each day until the end of vase life. To assess bacteria in stems, 0.5 g (∼2 cm length) end segments were excised from the stem ends and washed three times with distilled water. The explants were ground and diluted with 0.9% (v/v) normal saline. Bacterial colonies were enumerated after the incubation for 24 h at 37°C [30].
2.6 Bacterial isolation and identification
The proximal end segments (∼1 cm) were excised and surface‐sterilised with 70% ethanol. The explants were further cut into ∼20 small pieces and transferred into sterile tubes with 0.9% (w/v) normal saline (1 mL). The tubes were vortex‐mixed for 5 min. An aliquot (0.1 mL) was then serially diluted with sterile 0.9% (w/v) normal saline, spread on LB agar plates and incubated for 48 h at 37°C. Plates were sent to the Institute of Health and Environmental Sciences (Suzhou, China) for bacterial identification based on the 9th edition of Bergey's Manual of Determinative Bacteriology [41] and the Analytical Profile Index System (Biomerieux, Marcy l'Etoile, France). Molecular identification of bacteria was based on 16S rDNA gene sequences of the isolated bacteria. Genomic DNA was extracted from the dominant bacteria using the Genomic DNA Mini Preparation Kit (Beyotime Biotechnology, China). The 16S rDNAs of four isolates were PCR amplified using bacterial universal primers 27F (5′‐AGAGTTTGATCMTGGCTCAG‐3′) and 1492R (5′‐TACGGYTACCTTGTTACGACTT‐3′) and sequenced. The resulting 16S rDNA gene sequences compared with available 16S rDNA gene sequences from GenBank using the BLAST program (http://www.ncbi.gov/BLAST/) have been deposited in the GenBank database (accession no. KT896536–896539). Effect of the isolated stem‐end bacteria were tested on vase life of cut carnation flowers. Bacterial suspensions were diluted to 106 CFU/ml with distill water before used in vases as the preservation solution [28]. Vase life was calculated as above mentioned.
2.7 Antibacterial activity assay
The antibacterial activity of AgNPs at various concentrations was measured against the dominant bacterial strains in vitro. 0.1% (v/v) callus extracts were used as positive control. The AgNP solution (9.9 ml) or extracts (9.9 ml) was mixed with 0.1 ml bacterial suspensions (108 CFU/ml). Then, 0.1 ml aliquots were diluted with autoclaved distilled water to achieve ∼30–300 bacterial colonies on LB agar plate. Bacterial colonies were enumerated after the incubation for 48 h at 37°C. The antibacterial rate was defined by the equation to recognise the antibacterial effect: antibacterial rate (%) = (Nc −Nt ) × 100/Nc ; where Nc is average number of bacteria colonies for the control and Nt is that for the AgNP treatment [28].
2.8 Microscopic observation
The presence of bacteria was observed by scanning electron microscope (SEM) [28]. The AgNP pulse was applied at 250 mg/l for 1 h while control stems were pulsed with distilled water. Stem segments (3 mm long) were cut from the end of each stem using fresh razor blades on day 4 of the vase period. Each sample was immediately fixed in 4% (v/v) glutaraldehyde overnight. The ethanol‐CO2 critical point dried sample was coated with gold and examined by S‐4700 SEM (Hitachi, Japan) at 10 kV.
3 Results
3.1 Synthesis of AgNPs
The A. annua callus was induced from the leaf explants by 6‐BA 0.5 mg/l and NAA 1.0 mg/l in the MS medium. The white and yellowish callus was initiated from the leaf discs after 2 weeks (Figs. 1 a and b). When callus extracts were incubated with 1 mM AgNO3, the colour change was observed from pale green to brown in the extracts (Figs. 1 c and d). This colour change was mainly due to the silver ion reduction, which indicated the possible formation of stable AgNPs [42]. The absorption spectrum of the mixture showed a peak at 465 nm and the absorbance increased with increasing sunlight intensity from 4500 to 25,000 lx (Fig. 2 a). In contrast, no UV‐visible absorbance was occurred around 465 nm in the dark. Furthermore, UV‐visible absorbance increased with increasing time from 20 to 60 min (Fig. 2 b).
Fig. 1.

Callus induction from A. annua and colour intensity of the callus extracts incubated with 1 mM AgNO3
(a) Aseptic seedlings, (b) Callus culture, (c) Colour intensity at the beginning of the reaction, (d) Colour intensity after 60 min
Fig. 2.
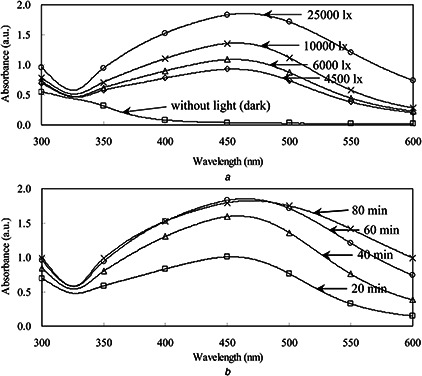
UV‐visible absorbance of AgNPs synthesised with the callus extracts
(a) Under different sunlight intensities (4500–25,000 lx), (b) With different reaction times
3.2 Characterisation of AgNPs
The shape and size of AgNPs was analysed using TEM and AFM (Figs. 3 and 4). From the TEM images, the nanoparticles were in spherical shape with a few irregularly shaped particles (Fig. 3). Most of the nanoparticles were scattered. The size of AgNPs varied from 2.1 to 45.2 nm. AFM studies have further confirmed the colloid size and morphology for the obtained AgNPs (Fig. 4). The two‐ and three‐dimensional topography of the AgNPs was shown in Figs. 4 a and b, revealing that the AgNPs were spherical and without aggregation. The line profile analysis showed the average grain size was 10.9 nm (Fig. 4 c), which is comparable to the results obtained by TEM observation. Fig. 4 d was a representation of the topography of an image cross section of the AFM picture.
Fig. 3.
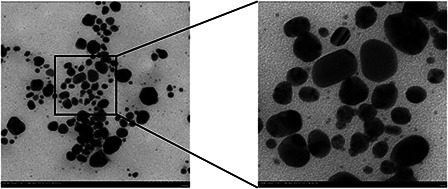
TEM analysis of AgNPs synthesised with the callus extracts and AgNO3 under sunlight intensity of 25,000 lx
Fig. 4.
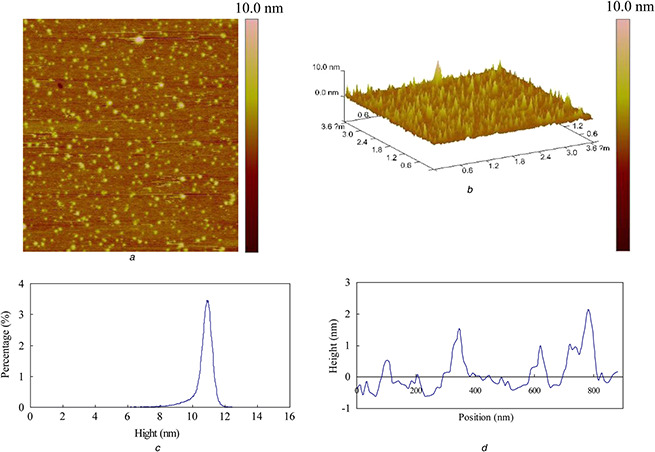
AFM analysis of AgNPs synthesised with the callus extracts and AgNO3 under sunlight intensity of 25,000 lx
(a) 2D image, (b) 3D image, (c) Peak distribution, (d) Height
The XRD pattern of AgNPs exhibited intense peaks for AgNPs in the whole spectrum of 2θ value ranging from 10 to 70 (Fig. 5 a). Diffraction peaks were observed at 2θ value of 38.05°, 44.10°, 64.40° and 77.33°, which corresponded to the (111), (200), (220) and (311) reflections of face‐centred silver (JCPDS no. 04‐0783). There were a few additional sharp unassigned peaks at 27.80°, 32.21°, 46.20°, 54.78° and 57.42°, respectively.
Fig. 5.
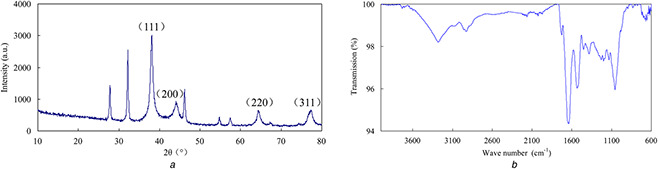
XRD pattern of AgNPs exhibited intense peaks for AgNPs in the whole spectrum of 2θ value ranging from 10 to 70 and FTIR spectra of AgNPs exhibited prominent peaks
(a) XRD pattern and, (b) FTIR spectra of the biosynthesised AgNPs
FTIR spectra of AgNPs exhibited prominent peaks at 3281, 2930, 1726, 1638, 1534, 1451, 1381, 1230, 1201, 1132 and 1056 cm−1 (Fig. 5 b). The peaks correspond to NH stretching, asymmetric stretching of C‐H bonds, C=O stretching bands, aliphatic C=C, amide II, CH bending, carboxyl group, amide III, C‐C stretching and C‐O group. The peaks assigned to amide II and III indicated the presence of the protein which may act as capping agents.
3.3 Vase life, water loss and ethylene production
AgNPs pulse treatments significantly extended the vase life of cut D. caryophyllus flowers at all concentrations (Table 1). The control flowers withered on day 5 while the AgNPs treatments had a similar phenomenon on day 9 as depicted in Fig. S1. The longest vase life was obtained by AgNPs pulse treatment at 250 mg/l, which resulted in 3.12 days longer vase life compared to the control. The amount of water loss in the control decreased after day 5 which was generally associated with bacterial plugging of stems. However, since then water loss in AgNP treatments has increased, which was consistent with the results of vase life (Fig. 6 a). On the other hand, the release of ethylene from control flowers exhibited a gradual increase, reaching the peak value around day 5 (Fig. 6 b). Ethylene production was markedly reduced by AgNPs treatment at 250 mg/l compared with the control.
Table 1.
Effect of stem‐end bacteria strains and 1‐h pulse treatment of the biosynthesised AgNPs on vase life of cut carnation flowers. Vase life data are means ± standard deviation of nine replicate stems
| Treatment | Vase life (days) | |
|---|---|---|
| Control | 5.79 ± 0.22 d | |
| AgNPs (mg/l) | 125 | 8.61 ± 0.46 e |
| 250 | 8.91 ± 0.46 e | |
| 500 | 8.57 ± 0.76 e | |
| Bacteria (106 CFU/ml) | M. oxydans | 2.57 ± 0.18 a |
| A. arilaitensis | 2.52 ± 0.17 a | |
| Straphylococcus equorum | 2.99 ± 0.16 b | |
| Kocuria sp. | 3.61 ± 0.26 c | |
Different letters indicate significant differences at P ≤ 0.05.
Fig. 6.
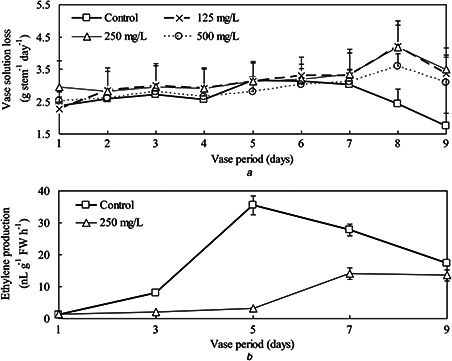
Water loss in AgNP treatments has increased, which was consistent with the results of vase life and the release of ethylene from control flowers exhibited a gradual increase
(a) Vase water loss and, (b) Ethylene production of cut carnation flowers with/without 1‐h AgNPs pulse treatment. Data are means ± standard deviation of nine replicate stems
3.4 Antibacterial activity of AgNPs
In the vase solution of control, bacterial counts increased rapidly from days 0 to 2 then reached a plateau, while there were no bacteria counted before day 6 in the AgNP pulse treatments (Fig. 7). Numbers of bacteria in the stem ends tended to increase during vase life, and the numbers were significantly higher in the control than in the NS pulse treatment (Fig. 7). Microscope observations showed that bacteria were observed in xylem vessels at the cut stem end of control on day 4. In contrast, few bacteria were evident in xylem vessels of flowers pulsed with 250 mg/l AgNPs (Fig. 8).
Fig. 7.
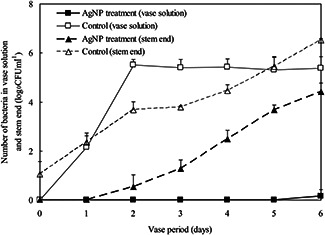
Changes in numbers of bacteria in vase solution and stem end of cut carnation flowers with/without 1‐h AgNP pulse treatment at 250 mg/l. Data are means ± standard deviation of three replications
Fig. 8.
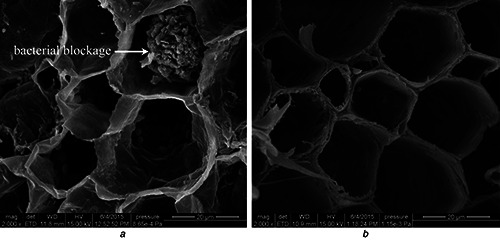
SEM observations of bacteria on the stem‐ends of cut carnation flowers
(a) Control, without AgNPs pulse before being stood into vase water and observed on day 4 of the vase period, (b) Treatment, flowers pulsed with 250 mg/l AgNPs for 1 h before being stood into vase water and observed on day 4 of the vase period
The four dominant bacterial strains identified from the stem ends were Microbacterium oxydans, Arthrobacter arilaitensis, Straphylococcus equorum and Kocuria sp. (Fig. S2). Placing those four strains into the vase water resulted in significant reductions in vase life (Table 1). The antibacterial effect of AgNPs was nearly 100% with 500 mg/l AgNPs against Straphylococcus equorum, 250 mg/l against M. oxydans and A. arilaitensis. However, AgNPs displayed weaker inhibitory effect against Kocuria sp. and only reached 61.84% at 500 mg/l (Table 2). The aqueous extracts of the calli at the concentration of 0.1% (v/v) used for AgNP synthesis had lower antibacterial activity (0.43–17.79%) to tested strains.
Table 2.
The antibacterial efficacy of AgNPs against four bacteria strains isolated from the stem‐end of cut carnation flowers. Data are means ± standard deviation of 3 replications
| Treatments | Antibacterial effect % | ||||
|---|---|---|---|---|---|
| M. oxydans | A. arilaitensis | S. equorum | Kocuria sp. | ||
| AgNPs (mg/l) | 125 | 67.79 ± 3.28 b | 21.25 ± 4.47 b | 33.08 ± 5.74 c | 42.40 ± 4.46 b |
| 250 | 97.59 ± 1.80 a | 99.67 ± 0.27 a | 72.82 ± 7.89 b | 57.89 ± 6.22 a | |
| 500 | 98.23 ± 0.67 a | 100.00 ± 0.00 a | 100.00 ± 0.00 a | 61.84 ± 6.34 a | |
| callus extracts 0.1% (v/v) | 5.18 ± 1.48 c | 15.40 ± 1.56 c | 17.79 ± 1.53 d | 0.43 ± 0.23 c | |
Different letters indicate significant differences at P ≤ 0.05.
The antibacterial rate (%) = (N c –N t) × 100/N c; where N c is average number of bacteria colonies for the control and N t is average number of bacteria colonies of the treatment.
4 Discussion
Senescence of carnation flowers is associated with the blockage of xylem vessels by microorganisms in the vase solution or in the stem end [24, 25]. Stem‐end bacteria caused not only the blockage with the loss of water from the flowers and stems, but also their decay products including enzymes and toxic compounds to accelerate senescence [23]. Inclusion of biocides in the vase water is a valid practice to inhibit bacterial growth and extend vase life of cut carnation flowers. Sodium dichloroisocyanuric acid at 50 mg/l, 1‐bromo‐3‐chloro‐5,5‐dimethylhydantoin at 12 mg available chlorine/l and HQC at 250 mg/l could extend the vase life of cut carnation from 6.0 to 6.6 days [43]. Macnish et al. [44] found that the vase life of cut carnation flowers was prolonged 2 days in vases containing ClO2 at 10 μl/l. However, effective concentrations of these biocides and metal salts can be toxic to flowers, human health and environment [45]. The AgNPs has potential for use in flower industry to prolong vase life [32]. Hamidimoghadam et al. [33] prolonged vase life of cut Pink Castellaro carnation from 7 to 9 days in vase solutions containing 10 mg/l AgNPs. Vase life of cut Tempo carnation was extended from 11 to 15 days by treating with 5 mg/l AgNPs [34]. In our present study, the pulse treatment of AgNPs at 250 mg/l could extend the vase life of cut carnations cv. ‘Green land’ from 5.8 to 8.9 days. We provided a simple and rapid procedure for biological synthesis of the applied AgNPs by using extracts of A. annua callus. The AgNPs synthesised with the extracts from A. annua leaves have been reported to have in vitro antibacterial activities against human pathogenic bacteria [13], antioxidant and corrosion inhibition potentials [14]. As far as we know, this is the first time for biologically synthesised AgNPs used for improving the postharvest quality of cut carnations.
Use of plant extract or plant biomass is a successful alternative to chemical and physical methods for the synthesis of AgNPs in an eco‐friendly manner [7]. Callus was used to synthesis AgNPs in tissue culture technique of C. papaya because it was easy to grow in a short period of time [16]. There are many examples for using callus extracts in the synthesis of AgNPs, such as callus extracts from C. roseus [17], M. sativa [18] and Citrullus colocynthis [46]. Callus or cell cultures, as an incessant source for plant biomass, have superior advantages over the plant origins. It overcomes the difficulties in cultivation of perennial plants, phytochemical variability at different geographical locations and avoids the cost of the harvesting and competition of nutrition [18]. In addition, mass of young callus may be metabolically active to synthesise various phytochemicals to reduce silver ions for AgNP synthesis. The leaf callus extracts from saltmarsh plants have been demonstrated to be more efficient in synthesis of the AgNPs than intact leaf extracts [15]. In our present study, we obtained AgNPs with the average grain size of 10.9 nm, which is smaller than the nanoparticles synthesised by leaf extracts of A. annua [13]. In addition to plant extract source and substrate concentration, other factors such as temperature, exposure time and light conditions have been known to affect the biosynthesis of AgNPs as well [38, 47]. Previous reports showed that sunlight could mediate the green synthesis of AgNPs using plant biomass [38, 48]. Our results indicated that light was responsible for biosynthesis of AgNPs and the conversion rate was influenced by sunlight intensity and exposure time. Previous studies have showed that A. annua extracts contain secondary metabolites including sesquiterpenoids, triterpenoids, flavonoids, coumarins, steroids, phenolics and lipids [12]. It was presumed that the flavonoids and phenolic acids in the extracts may act as reducing and capping agents [49]. Our FTIR spectra of AgNPs exhibited peaks which were found commonly in the nanoparticles synthesised by plant extracts [15], and indicated the presence of phenolics and proteins which may act as capping agents (Fig. 5 b). Although there are many studies of A. annua extracts with antimalarial, antibacterial and antitumour activities [50], the extracts of A. annua callus herein for biological synthesis of AgNPs are new industrial application of A. annua.
It has been reported that the essential oil of A. annua aerial parts inhibited remarkably the growth of tested Gram‐positive bacteria Enterococcus hirae [51]. Our results demonstrated that aqueous extracts of A. annua callus at the concentrations used for AgNP synthesis had no or slight antibacterial activity against four dominant bacterial strains isolated from the stem ends of cut carnation flowers (Table 1). However, the biosynthesised AgNPs had significant antibacterial activities against those bacterial strains. Of the strains tested, Kocuria sp. was isolated previously from seeds and endocarp of papaya fruits [52]. A. arilaitensis was isolated from leaf surfaces of Citrus paradise [53]. M. oxydans represented the major number of endophytic isolates in leaves of Chinese cabbage [54]. Staphylococcus equorum was culturable endophytic bacteria from Moso bamboo (Phyllostachys edulis) [55]. In our study, the number of bacteria in the stem ends increased exponentially in control group and the number in vase solutions peaked on day 2 (about 106 cfu/ml). We found that 250 mg/l AgNPs applied for 1 h almost completely killed the bacteria in vase solution. The significant inhibition on bacteria in stem ends was corroborated by SEM observations (Fig. 8). The bacterial population could lead to the vascular blockage and thereby low water uptake [56]. In our study, the amounts of vase water loss by the cut carnation increased upon AgNP treatment, indicating a possible relationship between water uptake and bacterial occlusions in the basal stem ends of cut carnation flowers. AgNPs application resulted in better biomass maintenance, suppression of bacterial growth and vascular blockage reduction, which increased the water uptake, maintained the turgidity of flowers and prolonged vase life finally [57]. The physiological role of AgNPs in extending the vase‐life of cut carnation flowers remains to be further explored.
5 Conclusions
In conclusion, a simple and rapid procedure for the biosynthesis of AgNPs using aqueous extracts of A. annua callus was developed. The stable and spherical shaped nanoparticles of average size ∼10 nm has been characterised using UV‐vis spectroscopy, TEM, AFM, XRD and FTIR. The pulse treatment of the AgNPs at 125–500 mg/l for 1 h significantly extended the vase life of cut carnation cv. Green Land compared with the control. The AgNP pulse significantly alleviated bacteria related blockage in the stem‐ends of cut carnations due to the strong antibacterial efficacy of AgNPs against the dominant bacteria in the stem‐ends of cut carnations. In addition, ethylene release of cut carnation flowers was inhibited in response to AgNP pulse. As far as we know, this is the first time the biologically synthesised AgNPs could be used as a promising preservative agent for improving the postharvest quality of cut carnations. With the improvement on callus multiplication and the optimisation of treatment process, it could be exploited for developing an effective biosynthesis of AgNPs in a large scale.
6 Acknowledgments
The authors are grateful to the Graduate Program of Higher Education in Jiangsu Province (grant no. CXLX13_84), Suzhou Scholar Program (grant no. 14317363) and the projects sponsored by the NSFC (grant no. 81273487), PAPD and SRF for ROCS (grant no. K513201011) for financial support of this work.
7 References
- 1. Wiley B. Sun Y. Mayers B. et al.: ‘Shape‐controlled synthesis of metal nanostructures: the case of silver’, Chem.‐Eur. J., 2005, 11, pp. 454 –463 [DOI] [PubMed] [Google Scholar]
- 2. Tavakoli A. Sohrabi M. Kargari A.: ‘A review of methods for synthesis of nanostructured metals with emphasis on iron compounds’, Chem. Pap., 2007, 61, pp. 151 –170 [Google Scholar]
- 3. Mokhtari N. Daneshpajouh S. Seyedbagheri S. et al.: ‘Biological synthesis of very small silver nanoparticles by culture supernatant of Klebsiella pneumonia: the effects of visible‐light irradiation and the liquid mixing process’, Mater. Res. Bull., 2009, 44, pp. 1415 –1421 [Google Scholar]
- 4. Kumar S.A. Abyaneh M.K. Gosavi S.W. et al.: ‘Nitrate reductase‐mediated synthesis of silver nanoparticles from AgNO3 ’, Biotechnol. Lett., 2007, 29, pp. 439 –445 [DOI] [PubMed] [Google Scholar]
- 5. Ahmad N. Sharma S. Alam M.K. et al.: ‘Rapid synthesis of silver nanoparticles using dried medicinal plant of basil’, Colloid Surface B, 2010, 81, pp. 81 –86 [DOI] [PubMed] [Google Scholar]
- 6. Philip D. Unni C.: ‘Extracellular biosynthesis of gold and silver nanoparticles using Krishna tulsi (Ocimum sanctum) leaf’, Physica E, 2011, 43, pp. 1318 –1322 [Google Scholar]
- 7. Park Y. Hong Y.N. Weyers A. et al.: ‘Polysaccharides and phytochemicals: a natural reservoir for the green synthesis of gold and silver nanoparticles’, IET Nanobiotechnol., 2011, 5, pp. 69 –78 [DOI] [PubMed] [Google Scholar]
- 8. Song J.Y. Kim B.S.: ‘Rapid biological synthesis of silver nanoparticles using plant leaf extracts’, Bioproc. Biosyst. Eng., 2009, 32, pp. 79 –84 [DOI] [PubMed] [Google Scholar]
- 9. Chandran S.P. Chaudhary M. Pasricha R. et al.: ‘Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract’, Biotechnol. Progr., 2006, 22, pp. 577 –583 [DOI] [PubMed] [Google Scholar]
- 10. Sathishkumara G. Gobinatha C. Karpagama K. et al.: ‘Phyto‐synthesis of silver nanoscale particles using Morinda citrifolia L. and its inhibitory activity against human pathogens’, Colloid Surface B, 2012, 95, pp. 235 –240 [DOI] [PubMed] [Google Scholar]
- 11. Naeem M. Idrees M. Singh M. et al.: ‘ Artemisia annua: a miraculous herb to cure malaria’, in Aftab T. Ferreira J.F.S. Khan M.M.A. et al. (Eds): ‘Artemisia annua‐pharmacology and biotechnology’ (Springer, Berlin Heidelberg, 2014), pp. 27 –49 [Google Scholar]
- 12. Bhakuni R.S. Jain D.C. Sharma R.P. et al.: ‘Secondary metabolites of Artemisia annua and their biological activity’, Curr. Sci., 2001, 80, pp. 35 –48 [Google Scholar]
- 13. Basavegowda N. Idhayadhulla A. Lee Y.R.: ‘Preparation of Au and Ag nanoparticles using Artemisia annua and their in vitro antibacterial and tyrosinase inhibitory activities’, Mater. Sci. Eng. C‐Mater., 2014, 43, pp. 58 –64 [DOI] [PubMed] [Google Scholar]
- 14. Johnson A.S. Obot I.B. Ukpong U.S.: ‘Green synthesis of silver nanoparticles using Artemisia annua and Sida acuta leaves extract and their antimicrobial, antioxidant and corrosion inhibition potentials’, J. Mater. Environ. Sci., 2014, 5, pp. 899 –906 [Google Scholar]
- 15. Nabikhan A. Kandasamy K. Raj A. et al.: ‘Synthesis of antimicrobial silver nanoparticles by callus and leaf extracts from saltmarsh plant, Sesuvium portulacastrum L.’, Colloid Surface B, 2010, 79, pp. 488 –493 [DOI] [PubMed] [Google Scholar]
- 16. Mude N. Ingle A. Gade A. et al.: ‘Synthesis of silver nanoparticles using callus extract of Carica papaya – a first report’, J. Plant. Biochem. Biot., 2009, 18, pp. 83 –86 [Google Scholar]
- 17. Barkat M.A. Mujeeb M. Samim M. et al.: ‘Biosynthesis of silver nanoparticles using callus extract of Catharanthus roseus var. alba and assessment of its antimicrobial activity’, Brit. J. Pharm. Res., 2014, 4, pp. 1591 –1603 [Google Scholar]
- 18. Hegazy H.S. Rabie G.H. Shaaban L.D. et al.: ‘Extracellular synthesis of silver nanoparticles by callus of Medicago sativa ‘, Life Sci. J., 2014, 11, pp. 1211 –1214 [Google Scholar]
- 19. Chernousova S. Epple M.: ‘Silver as antibacterial agent: ion, nanoparticle, and metal’, Angew. Chem. Int. Ed., 2013, 52, pp. 1636 –1653 [DOI] [PubMed] [Google Scholar]
- 20. Jo Y.K. Kim B.H. Jung G.: ‘Antifungal activity of silver ions and nanoparticles on phytopathogenic fungi’, Plant Dis., 2009, 93, pp. 1037 –1043 [DOI] [PubMed] [Google Scholar]
- 21. Park H.G. Kim S.H. Kim H.J. et al.: ‘A new composition of nanosized silica‐silver for control of various plant diseases’, Plant Pathol. J., 2006, 22, pp. 295 –302 [Google Scholar]
- 22. Put H.M.C.: ‘Micro‐organisms from freshly harvested cut flower stems and developing during the vase life of chrysanthemum, gerbera and rose cultivars’, Sci. Hort., 1990, 43, pp. 129 –144 [Google Scholar]
- 23. Salmond G.P.C.: ‘Secretion of extracellular virulence factors by plant pathogenic bacteria’, Annu. Rev. Phytopathol., 1994, 32, pp. 181 –200 [Google Scholar]
- 24. van Doorn W.G. Zagory D. de Witte Y. et al.: ‘Effects of vase‐water bacteria on the senescence of cut carnation flowers’, Postharvest Biol. Tec., 1991, 1, pp. 161 –168 [Google Scholar]
- 25. van Doorn W.G. de Witte Y. Harkema H.: ‘Effect of high numbers of exogenous bacteria on the water relations and longevity of cut carnation flowers’, Postharvest Biol. Tec., 1995, 6, pp. 111 –119 [Google Scholar]
- 26. Edrisi B. Sadrpoor A. Saffari V.R.: ‘Effects of chemicals on vase life of cut carnation (Dianthus caryophyllus L. ‘Delphi’) and microorganisms population in solution’, J. Ornam. Hortic. Plants, 2012, 2, pp. 1 –11 [Google Scholar]
- 27. Rahman M.M. Ahmad S.H. Lgu K.S.: ‘ Psidium guajava and Piper betle leaf extracts prolong vase life of cut carnation (Dianthus caryophyllus) flowers’, Sci. World J., 2012, 2012, pp. 1 –9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li H. Huang X. Li J. et al.: ‘Efficacy of nano‐silver in alleviating bacteria‐related blockage in cut rose cv. Movie Star stems’, Postharvest Biol. Tec., 2012, 74, pp. 36 –41 [Google Scholar]
- 29. Lü P. Cao J. He S. et al.: ‘Nano‐silver pulse treatments improve water relations of cut rose cv. Movie Star flowers’, Postharvest Biol. Tec., 2010, 57, pp. 196 –202 [Google Scholar]
- 30. Liu J. He S. Zhang Z. et al.: ‘Nano‐silver pulse treatments inhibit stem end bacteria on cut gerbera cv. Ruikou flowers’, Postharvest Biol. Tec., 2009, 54, pp. 59 –62 [Google Scholar]
- 31. Liu P. Ratnayake K. Joyce D.C. et al.: ‘Effects of three different nano‐silver formulations on cut Acacia holosericea vase life’, Postharvest Biol. Tec., 2012, 66, pp. 8 –15 [Google Scholar]
- 32. Liu J. Zhang Z. Joyce D.C. et al.: ‘Effects of postharvest nanosilver treatments on cut flowers’, Acta. Hortic., 2009, 847, pp. 245 –250 [Google Scholar]
- 33. Hamidimoghadam E. Rabiei V. Nabigol A. et al.: ‘Postharvest quality improvement of carnation (Dianthus caryophyllus L.) cut flowers by gibberellic acid, benzyl adenine and nano silver’, Agri. Commun., 2014, 2, pp. 28 –34 [Google Scholar]
- 34. Hashemabadi D.: ‘The role of silver nano‐particles and silver thiosulfate on the longevity of cut carnation (Dianthus caryophyllus) flowers’, J. Environ. Biol., 2014, 35, pp. 661 –666 [PubMed] [Google Scholar]
- 35. Zheng L.P. Zhang Z. Zhang B. et al.: ‘Antifungal properties of Ag‐SiO2 core‐shell nanoparticles against phytopathogenic fungi’, Adv. Mater. Res., 2012, 476–478, pp. 814 –818 [Google Scholar]
- 36. Zhang B. Zheng L.P. Li W.Y. et al.: ‘Stimulation of artemisinin production in Artemisia annua hairy roots by Ag‐SiO2 core‐shell nanoparticles’, Curr. Nanosci., 2013, 9, pp. 363 –370 [Google Scholar]
- 37. Murashige T. Skoog F.: ‘A revised medium for rapid growth and bio assays with tobacco tissue culture’, Physiol. Plantarum., 1962, 15, pp. 473 –497 [Google Scholar]
- 38. Zarchi A.A.K. Mokhtari N. Arfan M. et al.: ‘A sunlight‐induced method for rapid biosynthesis of silver nanoparticles using an Andrachnea chordifolia ethanol extract’, Appl. Phys. A, 2011, 103, pp. 349 –353 [Google Scholar]
- 39. Lü P. Cao J. He S. et al.: ‘Effects of nano‐silver treatment on vase life of cut rose cv. Movie Star flowers’, J. Food Agric. Environ., 2010, 8, pp. 1118 –1122 [Google Scholar]
- 40. Tanase K. Otsu S. Satoh S. et al.: ‘Expression levels of ethylene biosynthetic genes and senescence‐related genes in carnation (Dianthus caryophyllus L.) with ultra‐long‐life flowers’, Sci. Hortic., 2015, 183, pp. 31 –38 [Google Scholar]
- 41. Holt J.G. Krieg N.R. Sneath P.H.A. et al.: ‘Bergey's manual of determinative bacteriology’ (The Williams and Wilkins Co, Baltimore, 1994, 9th edn.) [Google Scholar]
- 42. Mulvaney P.: ‘Surface plasmon spectroscopy of nanosized metal particles’, Langmuir, 1996, 12, pp. 788 –800 [Google Scholar]
- 43. Jones R.B. Hill M.: ‘The effect of germicides on the longevity of cut flowers’, J. Am. Soc. Hortic. Sci., 1993, 118, pp. 350 –354 [Google Scholar]
- 44. Macnish A.J. Leonard R.T. Nell T.A.: ‘Treatment with chlorine dioxide extends the vase life of selected cut flowers’, Postharvest Biol. Tec., 2008, 50, pp. 197 –207 [Google Scholar]
- 45. Damunupola J.W. Joyce D.C.: ‘When is a vase solution biocide not, or not only, antimicrobial?’, J. Jpn. Soc. Hortic. Sci., 2006, 77, pp. 1 –18 [Google Scholar]
- 46. Satyavani K. Ramanathan T. Gurudeeban S.: ‘Green synthesis of silver nanoparticles by using stem derived callus extract of bitter apple (Citrullus colocynthis)’, Dig. J. Nanomater. Bios., 2011, 6, pp. 1019 –1024 [Google Scholar]
- 47. Amin M. Anwar F. Janjua M.R.S.A. et al.: ‘Green synthesis of silver nanoparticles through reduction with Solanum xanthocarpum L. berry extract: characterization, antimicrobial and urease inhibitory activities against Helicobacter pylori ’, Int. J. Mol. Sci., 2012, 13, pp. 9923 –9941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rastogi L. Arunachalam J.: ‘Sunlight based irradiation strategy for rapid green synthesis of highly stable silver nanoparticles using aqueous garlic (Allium sativum) extract and their antibacterial potential’, Mater. Chem. Phys., 2011, 129, pp. 558 –563 [Google Scholar]
- 49. Tamulya C. Hazarika M. Borahb S.Ch. et al.: ‘In situ biosynthesis of Ag, Au and bimetallic nanoparticles using Piper pedicellatum C.DC: Green chemistry approach’, Colloid Surface B, 2013, 102, pp. 627 –634 [DOI] [PubMed] [Google Scholar]
- 50. Sendi J.J. Khosravi R.: ‘Recent developments in controlling insect, acari, nematode, and plant pathogens of agricultural and medical importance by Artemisia annua L. (Asteraceae)’, in Aftab T. Ferreira J.F.S. Khan M.M.A. et al. (Eds): ‘Artemisia annua‐pharmacology and biotechnology’ (Springer, Berlin Heidelberg, 2014), pp. 229 –247 [Google Scholar]
- 51. Juteau F. Masotti V. Bessière J.M. et al.: ‘Antibacterial and antioxidant activities of Artemisia annua essential oil’, Fitoterapia, 2002, 73, pp. 532 –535 [DOI] [PubMed] [Google Scholar]
- 52. Krishnan P. Bhat R. Kush A. et al.: ‘Isolation and functional characterization of bacterial endophytes from Carica papaya fruits’, J. Appl. Microbiol., 2012, 113, pp. 308 –317 [DOI] [PubMed] [Google Scholar]
- 53. Izhaki I. Fridman S. Gerchman Y. et al.: ‘Variability of bacterial community composition on leaves between and within plant species’, Curr. Microbiol., 2013, 66, pp. 227 –235 [DOI] [PubMed] [Google Scholar]
- 54. Haque M.A. Lee J.H. Cho K.M.: ‘Endophytic bacterial diversity in Korean kimchi made of Chinese cabbage leaves and their antimicrobial activity against pathogens’, Food Control, 2015, 56, pp. 24 –33 [Google Scholar]
- 55. Yuan Z. Liu F. Zhang G.: ‘Isolation of culturable endophytic bacteria from Moso bamboo (Phyllostachys edulis) and 16S rDNA diversity analysis’, Arch. Biol. Sci., 2015, 00, p. 63 [Google Scholar]
- 56. van Doorn W.G.: ‘Water relations of cut flowers’, Hort. Rev., 1997, 18, pp. 1 –85 [Google Scholar]
- 57. Hassan F.A.S. Ali E.F. El‐Deeb B.: ‘Improvement of postharvest quality of cut rose cv. ‘First Red’ by biologically synthesized silver nanoparticles’, Sci. Hortic., 2014, 179, pp. 340 –348 [Google Scholar]


