Abstract
Here, the authors report a rapid, simple, and eco‐friendly process for synthesis of Bi2 O3 nano‐needles. Dioscorea alata tuber extract was used as both reducing and capping agent for the first time. These nanoparticles were characterised by X‐ray diffraction, field emission scanning electron microscope, and Fourier transform infrared (FTIR) spectrometry, the nano‐structured Bi2 O3 needles have an average diameter of 158 nm with the lengths in the range of 1–3 μm. CLSI M27‐A2 standard was followed for evaluation of anti‐fungal activity. Bi2 O3 nano‐needles show remarkable activity against Candida albicans. It exhibits four time greater activity than bulk Bi2 O3 powder and two time greater activity than itraconazole, which makes it a potent anti‐fungal drug.
Inspec keywords: bismuth compounds, nanoparticles, X‐ray diffraction, field emission scanning electron microscopy, Fourier transform infrared spectra, drugs, nanomedicine, biomedical materials, nanofabrication
Other keywords: nanoneedles, antifungal activity, nanoparticles, X‐ray diffraction, field emission scanning electron microscope, Fourier transform infrared spectrometry, CLSI M27‐A2 standard, Candida albicans, itraconazole, antifungal drug, Bi2 O3
1 Introduction
Bismuth is known since ancient times, in spite of its location amid toxicity in periodic table; bismuth and its compounds are considerably harmless [1]. Many bismuth compounds are less toxic than that of sodium chloride [2]. This makes bismuth unique among the heavy metals and earned the status of ‘green element’ [1]. Bismuth subsalicylate, for example, is used as an antidiarrhoeal agent to treat nauseas, vomiting, and stomach pain [3]. Bismuth oxychloride is used to impart silver sheen to personal products and cosmetics. It is marketed as BIRON powder which has potent applications in surgical procedures.
Bismuth trioxide (Bi2 O3) is an important commercial oxide of bismuth. It is a precursor for the preparation of other bismuth compounds including chemical reagents [4]. Bi2 O3 shows a distinctive polymorphism including following solid phases α‐Bi2 O3 (monoclinic), β‐Bi2 O3 (tetragonal), γ‐Bi2 O3 (body centred cubic), and ω‐Bi2 O3 (triclinic) [5]. α‐Bi2 O3 is poorly water soluble that carries surface hydroxyl group [6] and relatively stable at room temperature. Bi‐O bonds are predominantly ionic as α‐Bi2 O3 is a basic oxide [7]. It has a potential application in the field of biomedicine, as it is used in dental materials to make them more opaque to X‐rays than surrounding tooth structure [8]. Bi2 O3 is a major component in the AnuSol cream which is used as antiseptic, astringent, and emollient [9]. It is used as a topical homeostatic for palatal wounds may be an option when performing free soft tissue grafts [10]. It is also employed as a potential agent for the treatment of infections caused by Helicobacter pylori [11]. The targets for bio‐coordination of bismuth compounds are non‐DNA sites, offering new opportunities for novel mechanism of action for treatment of cancer [12].
Nanoparticles possess large surface area and therefore increase interaction with biological targets. They have unique physical and chemical properties than their bulk counterparts, hence can be incorporated in the novel formulations. As Bi2 O3 nanoparticles are non‐toxic to human tissue [13], it can be used for temperature sensing, dual‐modal imaging, and drug delivery [14]. Bi2 O3 nano‐structures are used as an anti‐fungal agent [15], anti‐bacterial agents [16], anti‐cancer agent [17] etc.
For synthesis of Bi2 O3 nanostructure, various physical and chemical methods are employed which shows numerous morphologies [18].
Plant extract can be used as reducing and stabilising agents for synthesis of metal and metal oxide nanoparticles. The way to develop a green process is to adapt benign synthesis approach that used non‐toxic reaction precursors and mild reaction condition [19]. Plants are capable of generating a wide variety of advanced nano‐structures matching the sophistication of current engineered materials, wherein plant biomolecules mediate the safest and most cost‐effective, large‐scale production of biocompatible nanoparticles. Water‐soluble plant metabolites (e.g. alkaloids, phenolic compounds, flavonoids, terpenoids, and catechins) are responsible for the reduction [20]. Green route helps to minimise or eliminate the harmful polluting substance in synthesis of nano‐materials.
Dioscorea is a genus of flowering plants with over 600 species in the family Dioscoreaceae. Dioscorea alata is one such edible species which has the highest yield among all Dioscorea species and can be stored for relatively longer period [21]. It is a rich source of phytochemical such as flavonoids, phenolics, alkaloids, saponin etc. and is also rich in vitamin and mineral contents [22]. As Dioscorea species are rich in polyphenolic content and thus can be used for bio‐reduction of metal to nanoparticles [23].
Uddin et al. [24] have successfully synthesised Bi2 O3 nanoparticles by microbial synthesis using plant pathogenic fungus Fusarium oxysporum. Aguirre et al. [25] have synthesised β‐Bi2 O3 by using tannic acid as a reduction agent. All these processes require complex procedures and long incubation time though synthesised by a green route. On the other hand, Karnan et al. [26] have successfully synthesised Bi2 O3 nanoparticles by green route, but the incubation is time‐consuming and annealing temperature are relatively high. So, there is need to develop a simple, fast, and purely eco‐friendly process for synthesis of stable α‐Bi2 O3 nanoparticles. In this paper, we are first time reporting the synthesis of α‐Bi2 O3 using D. alata tuber extract. D. alata tuber extract is employed as both reducing and capping agent, for synthesis of α‐Bi2 O3 nanoparticles. The process is relatively simple, fast, and truly eco‐friendly which can be easily scale up.
2 Materials and method
2.1 Chemicals
Bismuth nitrate (Bi(NO3)3 ·5H2 O), bismuth trioxide (Bi2 O3), barium sulphate (BaSO4), nitric acid (HNO3), and dimethyl sulfoxide (DMSO) were procured from sd fine‐chem India. Itraconazole was procured from Sigma‐Aldrich and Liquid RPMI‐1640 medium supplemented with l ‐glutamine was purchased from HiMedia.
2.2 Collection of plant material
D. alata tubers were collected from field and identified in Botany Department, Shivaji University, Kolhapur, India.
2.3 Preparation of plant extract
D. alata tubers were peeled off and dice into small pieces which were dried in hot air oven, then pulverised, and stored at room temperature until further use. For preparation of extract, the dried powder (5 g) was weighed and transferred to 500 ml Erlenmeyer flask containing 100 ml deionised water, mixed using magnetic stirrer for 10 min and then transferred to preheated water‐bath at 80°C and incubated for 15 min. The extract was stained using sieve and then centrifuged at 8000 rpm for 15 min and the supernatant was filtered through Whatman No. 1 filter paper. Clear filtrate was collected and used for synthesis.
2.4 Synthesis of Bi2 O3 nano‐needles
A known quantity of 2.425 g (0.1 M) of bismuth nitrate was transferred to culture bottles containing 10% of 50 ml nitric acid. The solution was mixed thoroughly for 15 min using a magnetic stirrer. About 50 ml of extract was added drop wise with constant stirring. The solution turns from colourless to light brown without forming precipitate (Fig. 1 c). After mixing for 20 min, the culture bottles were capped and autoclaved at 121°C and 15 lb for 21 min. After autoclaving, the colour of the solution changes to light yellow forming white colour precipitate (Fig. 1 d). The solution was allowed to cool at room temperature where the solution colour changes to colourless, the white colour precipitate was isolated by centrifugation. The obtained powder was washed thrice with distilled water and ethanol separately. Then the white precipitate was annealed at 350°C for 3 h in muffle furnace. Fig. 1 shows various stages of synthesis. The overall synthesis process was completed in <4 h. The resultant yellow colour powder was used for further characterisations.
Fig. 1.

Different stages in synthesis of α‐Bi2 O3 nano‐needles
(a) D. alata extract, (b) Solution of bismuth nitrate and nitric acid, (c) Mixture of D. alata extract and bismuth nitrate (a +b = light brown), (d) Mixture after immediate autoclaving (yellow), (e) Mixture after 8 h of cooling at room temperature autoclaving (colourless)
2.5 Characterisation of Bi2 O3 nano‐needles
The synthesised nanoparticles were characterised by X‐ray diffraction (XRD) measured on Brunker AXS D2 phaser diffractometer using Cu Kα radiation (k = 1.5406 Å). Dynamic light scattering measurement was done by using Malvern Instruments Zetasizer Nano ZS‐90 to obtain size distribution of the particle. For surface morphologies and particle size investigation, field emission scanning electron microscope (FE‐SEM) by a Mira‐3, Tescan, was employed. Fourier transformed infrared (FTIR) spectra were measured by Shimadzu FTIR spectrophotometer.
2.6 Anti‐fungal activity
Anti‐fungal activity of synthesised α‐Bi2 O3 nano‐needles against Candida albicans was evaluated. For comparison, the anti‐fungal activity of bulk Bi2 O3 powder and itraconazole was also evaluated. C. albicans 3471 strain was procured from NCIM. For anti‐candida activity, the inoculum preparation for micro‐dilution was performed in accordance with the CLSI document M27‐A2 [27]. C. albicans was subcultured on Sabroud's dextrose agar incubated at 35°C for 48 h. Candida cells were recovered and suspended in 10 ml saline. The turbidity was adjusted according to 0.5 McFarland standard (1 × 106 to 5 × 106 CFU/ml) according to protocol [27]. Suspension was diluted thousand folds with RPMI media to give final inoculum of (1 × 103 to 5 × 103 CFU/ml). The minimum inhibition concentration (MIC) was determined by micro‐dilution test (CLSI M27‐A2) [27]. For preparation of the drug suspension, Bi2 O3 nano‐needles and bulk Bi2 O3 powder were dissolved in 10% HNO3, and the itraconazole was dissolved in DMSO. The broth microdilution test was performed by using sterile, disposable, multiwell microdilution plates (96 U‐shaped wells). In the first row, 100 µl Bi2 O3 nano‐needle suspension (ranging from 16 to 0.0313 µg/ml) was added. In the second row, 100 µl normal Bi2 O3 suspension (ranging from 16 to 0.0313 µg/ml) was added. In the third row, 100 µl itraconazole suspension (ranging from 16 to 0.0313 µg/ml) was added in decreasing concentration. Column 11 (drug‐free) is growth control well and in row 12, only medium was added to check its sterility. About 100 µl of microbial suspension was added in every well except column 12. About 100 µl media was added in every well. The microtiter plate was covered and incubated at 35°C for 48 h. The assay was performed in triplicate.
3 Result and discussion
3.1 XRD analysis
XRD pattern (Fig. 2) of the synthesised nanoparticles was recorded to identify the phase of bismuth oxide (Bi2 O3) and to evaluate the crystalline size. The observed peaks were identified with α phase of bismuth oxide using JCPDS data (card no. 00‐014‐0699) which reveals high phase purity of α‐Bi2 O3. The grain size was estimated from the most intense peak (0 1 2) using Scherrer's formula, D = 0.9λ /β cos θ, where D is the grain size, λ the wavelength of X‐rays, β the full width of peak at half maximum in radian, and θ the Bragg angle [28]. The average grain size was found to be 41.71 nm.
Fig. 2.
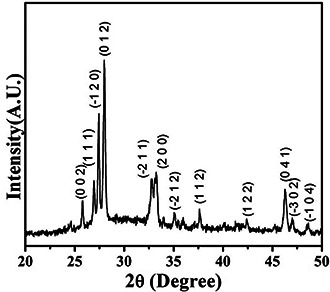
XRD pattern of synthesised α‐Bi2 O3 nano‐needles
3.2 Field emission scanning electron microscope
Morphology and particle size of the nano‐needles were investigated by FE‐SEM. The FE‐SEM image shown in Fig. 3 reveals the formation of the numerous straight nano‐needles, randomly orientated in the sample, with typical lengths of 1–3 μm. The FE‐SEM image of nano‐needles was quantitatively analysed using ImageJ 1.50i software. These measurements showed that the Bi2 O3 nano‐needles are 130–200 nm in diameter and have average diameter of 158 nm with standard deviation of 0.02. Crystallite size of 42 nm, calculated using X‐ray crystallographic analysis, was smaller than the average needle diameter of 158 nm obtained from the FE‐SEM. The average length was 1.96 μm with standard deviation of 0.37. The individual needles possess uniform diameters throughout their lengths with an average aspect ratio of generally >10 nanoparticles of diameter 185 nm and length >1 μm. The morphology of the synthesised material match with the morphology of α‐Bi2O3 synthesised by the other method [28, 29].
Fig. 3.
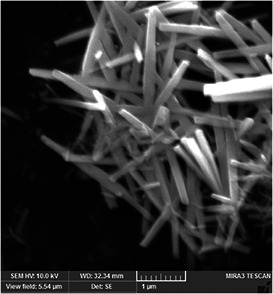
FE‐SEM of α‐Bi2 O3 nano‐needles
3.3 FTIR analysis
The chemical groups and characteristics bands of nano‐needles are revealed by FTIR spectra shown in Fig. 4. The major peak observed at 511 cm−1 is assigned for bending vibration of Bi–O [30]. Bi‐O‐Bi peak is located at 588 cm−1 and the peak observed at 630 cm−1 is assigned for vibration of the Bi–O non‐bridging oxygen. The peak located at 646 cm−1 is for Bi–O and at 846 cm−1 is for Bi–O–C [31]. The peak at 1019 and 1384 cm−1 are assigned for the Bi=O bending vibration [32] and Bi–N, respectively.
Fig. 4.
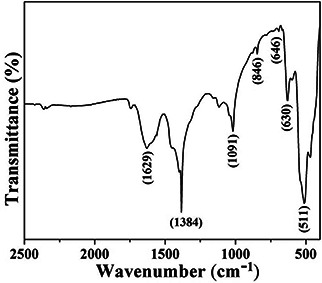
FTIR spectra of α‐Bi2 O3 nano‐needles
3.4 Anti‐fungal activity
After incubation, the microtiter plate was observed for the presence of viable growth as shown in Fig. 5. The microdilution wells are scored with the aid of a reading mirror the growth in each well is compared with that of the growth control well. The MIC obtained for all three compounds are shown in Table 1. Bi2 O3 nano‐needles require lowest concentration for inhibition of C. albicans as compared to bulk Bi2 O3 powder. Thus, the nanoformulation is four times more efficient than bulk Bi2 O3 powder. Also, Bi2 O3 nano‐needles are two times more efficient than itraconazole which indicates that it is a potent anti‐fungal agent.
Fig. 5.
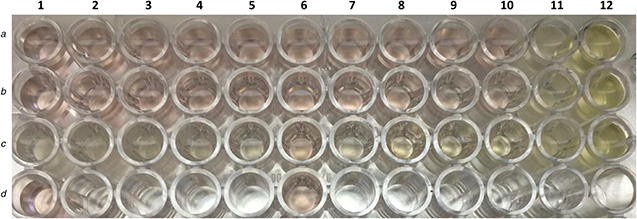
Anti‐candida activity. Row A, Bi2 O3 nano‐needles; row B, bulk Bi2 O3 powder; row C, itraconazole; row D, 10% HNO3; column 11, positive control; column 12, sterility control
Table 1.
MIC of all three compounds
| Compound | MIC, µg/ml |
|---|---|
| Bi2 O3 nano‐needles | 0.0625 |
| Bi2 O3 | 0.250 |
| Itraconazole | 0.125 |
4 Conclusion
In summary, Bi2 O3 nano‐needles were successfully synthesised within 4 h by simple, eco‐friendly, and green route using D. alata tuber extract for the first time. This system overcomes the disadvantage of the conventional methods such as long incubation time, very high temperature and pressure conditions, complex and expensive equipments, and time‐consuming reactions, reported in the previous literature [33, 34, 35]. D. alata tuber extract is a promising reducing and capping agent in synthesis of nanoparticles, which also maintains mild condition in the process. Bi2 O3 nano‐needles are found to be more efficient anti‐fungal agent than Bi2 O3 bulk powder and itraconazole.
5 Acknowledgments
We would like to thank Dr D.K. Gaikwad, Head of Botany Department, Shivaji University, Mr M.V. Patgaonkar, Head of Biotechnology Department, KIT's College of Engineering Kolhapur, Dr A.A. Kulkarni, Biotechnology Department, KIT's College of Engineering Kolhapur, Dr P.S. Patil and P.P. Waifalkar, Shivaji University Kolhapur, for their immense support and useful inputs. We would also like to thank D.Y. Patil Medical College, Kolhapur for donating itraconazole.
6 References
- 1. Mohan R.: ‘Nature bismuth’, Nat. Chem., 2010, 2, p. 336 [DOI] [PubMed] [Google Scholar]
- 2. Suzuki H. Komatsu N. Ogawa T. et al.: ‘Organobismuth chemistry’ (Elsevier Science, Amsterdam, 2001) [Google Scholar]
- 3. Figueroa‐Quintanilla D. Salazar‐Lindo E. Sack R. B. et al.: ‘A controlled trial of bismuth subsalicylate in infants with acute watery diarrheal disease’, N. Engl. J. Med., 1993, 328, (23), pp. 1653 –1658 [DOI] [PubMed] [Google Scholar]
- 4. Kelly Z. Ojebuoboh F.: ‘Producing bismuth trioxide and its application in fire assaying’, JOM, 2002, 54, (4), pp. 42 –45 [Google Scholar]
- 5. Mehring M.: ‘From molecules to bismuth oxide‐based materials: potential homo‐ and heterometallic precursors and model compounds’, Coord. Chem. Rev., 2007, 251, (7–8), pp. 974 –1006 [Google Scholar]
- 6. Cox P.A.: ‘The elements: their origin, abundance, and distribution’ (Oxford University Press, Oxford, 1989) [Google Scholar]
- 7. Earnshaw A. Greenwood N.N.: ‘Chemistry of the elements’ (Pergmon Press Ltd, Oxford, 1984, 1st edn.) [Google Scholar]
- 8. Josette C.: ‘Color stability of white mineral trioxide aggregate in contact with hypochlorite solution’, J. Endod., 2014, 40, (3), pp. 436 –440 [DOI] [PubMed] [Google Scholar]
- 9.‘AnuSol Cream’. Available at https://www.medicines.org.uk/emc/medicine/7159, accessed October 2016
- 10. Kim S.H. Tramontia V.A. Papalexiou V. et al.: ‘Bismuth subgallate as a topical hemostatic agent at palatal donor sites’, Quintessence Int., 2010, 41, (8), pp. 645 –649 [PubMed] [Google Scholar]
- 11. Ketata M. Desjardins Y. Ratti C.: ‘Effect of liquid nitrogen pretreatments on osmotic dehydration of blueberries’, J. Food Eng., 2013, 116, (1), pp. 202 –212 [Google Scholar]
- 12. Tiekink E.: ‘Antimony and bismuth compounds in oncology’, Crit. Rev. Oncol. Hematol., 2002, 42, (3), pp. 217 –224 [DOI] [PubMed] [Google Scholar]
- 13.‘Bismuth trioxide toxicology’. Available at http://digitalfire.com/4sight/hazards/ceramic_hazard_bismuth_trioxide_toxicology_352.html, accessed October 2016
- 14. Zhu H. Li Y. Qiu R. et al.: ‘Responsive fluorescent Bi2O3@PVA hybrid nanogels for temperature‐sensing, dual‐modal imaging, and drug delivery’, Biomaterials, 2012, 33, (10), pp. 3058 –3069 [DOI] [PubMed] [Google Scholar]
- 15. Hernandez‐Delgadillo R. Velasco‐Arias D. Martinaz‐Sanmiquel J.J. et al.: ‘Bismuth oxide aqueous colloidal nanoparticles inhibit Candida albicans growth and biofilm formation’, Int. J. Nanomedicine, 2013, 8, pp. 1645 –1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aggrawal S. Chauhan I. Mohanty P.: ‘Immobilization of Bi2 O3 nanoparticles on the cellulose fibers of paper matrices and investigation of its antibacterial activity against E. coli in visible light’, Mater. Express, 2015, 5, (5), pp. 429 –436 [Google Scholar]
- 17. Sebastian A. Anandkumar B.S. Mohan C.D. et al.: ‘Preparation and use of combustion‐derived Bi2 O3 for the synthesis of heterocycles with anti‐cancer properties by Suzuki‐coupling reactions’, RSC Adv., 2014, 4, (94), pp. 52181 –52188 [Google Scholar]
- 18. Huang Q. Zhang S. Cai C. et al.: ‘β‐ and α‐Bi2 O3 nanoparticles synthesized via microwave‐assisted method and their photocatalytic activity towards the degradation of rhodamine B’, Mater. Lett., 2011, 65, (6), pp. 988 –990 [Google Scholar]
- 19. Korbekandi H. Iravani S.: ‘Biological synthesis of nanoparticles using algae’, in Rai M. Posten C., (Eds.): ‘Green biosynthesis of nanoparticles: mechanisms and applications’ (CABI, Wallingford, UK, 2013), pp. 53 –60 [Google Scholar]
- 20. Mohammadinejad R. Karimi S. Iravani S. et al.: ‘Plant‐derived nanostructures: types and applications’, Green Chem., 2016, 18, pp. 20 –52 [Google Scholar]
- 21. Faustina Dufie W.M. Oduro I. Ellis W.O. et al.: ‘Potential health benefits of water yam (Dioscorea alata)’, Food Funct., 2013, 4, pp. 1496 –1501 [DOI] [PubMed] [Google Scholar]
- 22. Okwu D.E. Ndu C.U.: ‘Evaluation of the phytonutrients, mineral and vitamin contents of some varieties of yam (Dioscorea sp.)’, Int. J. Mol. Med. Adv. Sci., 2006, 2, (2), pp. 199 –203 [Google Scholar]
- 23. Mittal A.K. Chisti V.K. Banerjee U.C.: ‘Synthesis of metallic nanoparticles using plant extracts’, J. Biotech Adv., 2013, 31, (2), pp. 346 –356 [DOI] [PubMed] [Google Scholar]
- 24. Uddin I. Adhynthaya S. Syed A.: ‘Structure and microbial synthesis of sub‐10 nm Bi2 O3 nanocrystals’, J. Nanosci. Nanotechnol. 2008, 8, (8), pp. 1 –5 [DOI] [PubMed] [Google Scholar]
- 25. Aguirre F.M.A. Becerra R.H.: ‘New synthesis of bismuth oxide nanoparticles Bi2 O3 assisted by tannic acid’, Appl. Phys. A, 2015, 119, (3), pp. 909 –915 [Google Scholar]
- 26. Karnan T. Selvakumar S.A.S. Adinaveen T. et al.: ‘Visible light induced photocatalytic degradation of azo dye by Bi2 O3 nanoparticles synthesized using greener route’, Int. J. Sci. Eng. Res., 2016, 7, p. 266 [Google Scholar]
- 27. M27‐A2 : ‘Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard – second edition’, 2002.
- 28. Jiang H.Y. Liu G. Li P. et al.: ‘Nanorod‐like α‐Bi2 O3: a highly active photocatalyst synthesized using g‐C3 N4 as a template’, RSC Adv., 2014, 4, (98), pp. 55062 –55066 [Google Scholar]
- 29. Lu H. Wang S. Zhao L. et al.: ‘Surfactant‐assisted hydrothermal synthesis of Bi2 O3 nano/microstructures with tunable size’, RSC Adv., 2012, 2, (8), pp. 3374 –3378 [Google Scholar]
- 30. Hazra S. Mandal S. Ghosh A.: ‘Properties of unconventional lithium bismuthate glasses’, Phys. Rev. B, 1997, 56, (13), p. 8021 [Google Scholar]
- 31. Pascuta P. Pop L. Rada S. et al.: ‘The local structure of bismuth germanate glasses and glass ceramics doped with europium ions evidenced by FT‐IR spectroscopy’, Vib. Spectrosc., 2008, 48, (2), p. 281 [Google Scholar]
- 32. Lines M.E. Miller A.E. Nassau K. et al.: ‘Absolute Raman intensities in glasses: II. Germania‐based heavy metal oxides and global criteria’, J. Non‐Cryst. Solids, 1987, 89, (1–2), pp. 163 –180 [Google Scholar]
- 33. Oudghiri‐Hassani H. Rakass S. Wadaani F.T.A.: ‘Synthesis, characterization and photocatalytic activity of α‐Bi2 O3 nanoparticles’, J. Taibah Univ. Sci., 2015, 9, (4), pp. 508 –512 [Google Scholar]
- 34. Madler L. Pratsinis S.E.: ‘Bismuth oxide nanoparticles by flame spray pyrolysis’, J. Am. Ceram. Soc., 2002, 85, (7), pp. 1713 –1718 [Google Scholar]
- 35. Huang X. Zhang W. Tan Y.: ‘Facile synthesis of rod‐like Bi2 O3 nanoparticles as an electrode material for pseudocapacitors’, Ceram. Int., 2016, 42, (1), pp. 2099 –2105 [Google Scholar]


