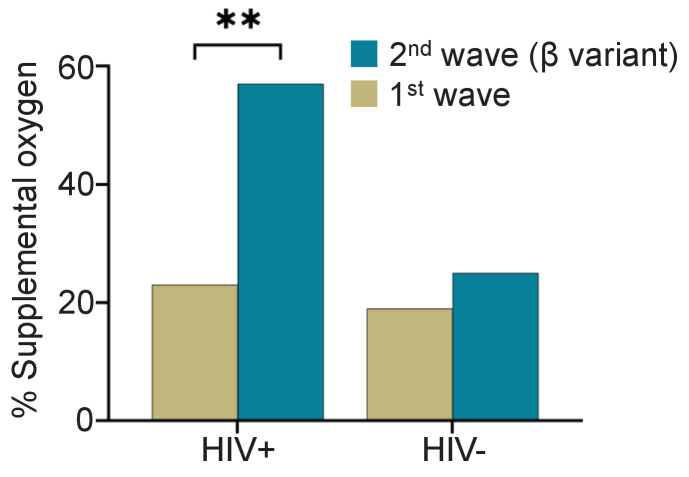
Figure 1. Fraction of PLWH and HIV-negative participants requiring supplemental oxygen during the first and the Beta variant dominated second infection waves.
p=0.0025 by Fisher’s Exact test.
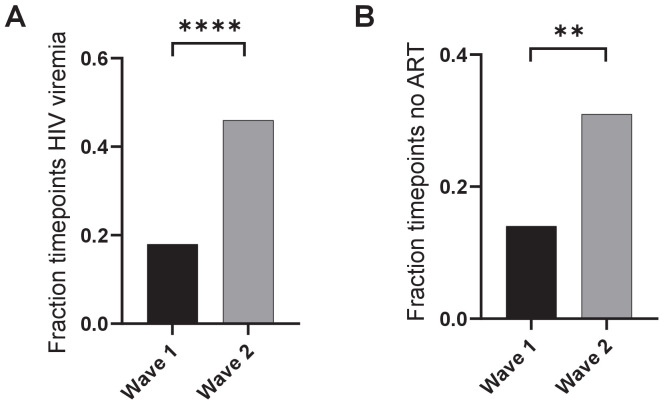
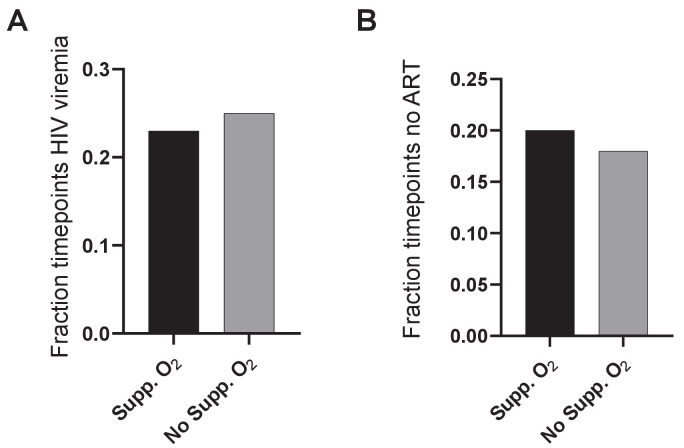
Figure 1—figure supplement 1. Viremia and ART in PLWH in wave 1 versus wave 2.
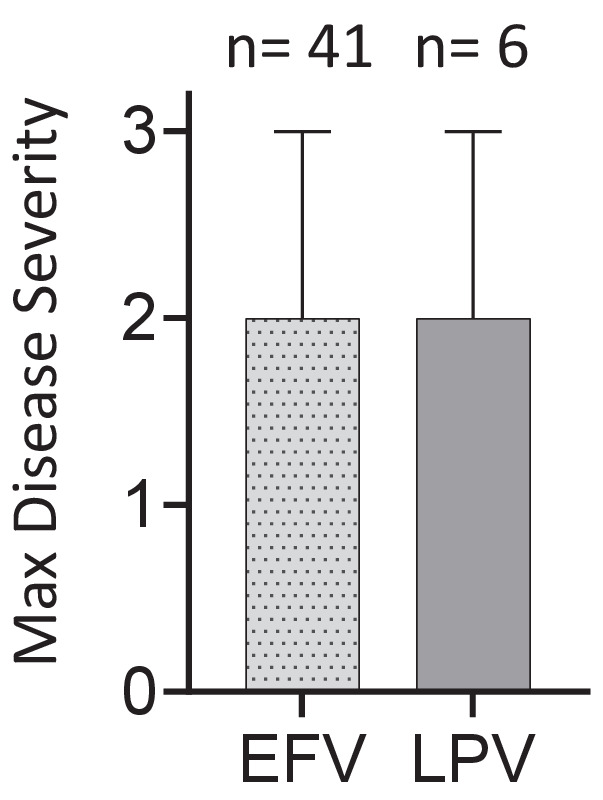
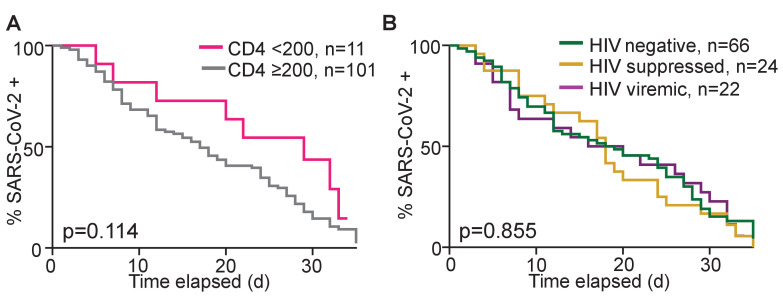
Figure 1—figure supplement 2. Effect of ART regimen on disease severity.