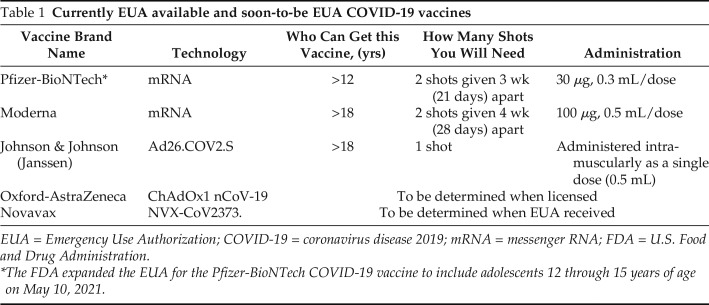
Table 1.
Currently EUA available and soon-to-be EUA COVID-19 vaccines
| Vaccine Brand Name | Technology | Who Can Get this Vaccine, (yrs) | How Many Shots You Will Need | Administration |
|---|---|---|---|---|
| Pfizer-BioNTech* | mRNA | >12 | 2 shots given 3 wk (21 days) apart | 30 μg, 0.3 mL/dose |
| Moderna | mRNA | >18 | 2 shots given 4 wk (28 days) apart | 100 μg, 0.5 mL/dose |
| Johnson & Johnson (Janssen) | Ad26.COV2.S | >18 | 1 shot | Administered intramuscularly as a single dose (0.5 mL) |
| Oxford-AstraZeneca | ChAdOx1 nCoV-19 | To be determined when licensed | ||
| Novavax | NVX-CoV2373. | To be determined when EUA received | ||
EUA = Emergency Use Authorization; COVID-19 = coronavirus disease 2019; mRNA = messenger RNA; FDA = U.S. Food and Drug Administration: .
The FDA expanded the EUA for the Pfizer-BioNTech COVID-19 vaccine to include adolescents 12 through 15 years of age on May 10, 2021.