Supplemental Digital Content is available in the text
Keywords: coronavirus disease 2019, mortality, sex
Abstract
Although the number of deaths due to coronavirus disease 2019 (COVID-19) is higher in men than women, prior studies have provided limited sex-stratified clinical data.
We evaluated sex-related differences in clinical outcomes among critically ill adults with COVID-19.
Multicenter cohort study of adults with laboratory-confirmed COVID-19 admitted to intensive care units at 67 U.S. hospitals from March 4 to May 9, 2020. Multilevel logistic regression was used to evaluate 28-day in-hospital mortality, severe acute kidney injury (AKI requiring kidney replacement therapy), and respiratory failure occurring within 14 days of intensive care unit admission.
A total of 4407 patients were included (median age, 62 years; 2793 [63.4%] men; 1159 [26.3%] non-Hispanic White; 1220 [27.7%] non-Hispanic Black; 994 [22.6%] Hispanic). Compared with women, men were younger (median age, 61 vs 64 years, less likely to be non-Hispanic Black (684 [24.5%] vs 536 [33.2%]), and more likely to smoke (877 [31.4%] vs 422 [26.2%]). During median follow-up of 14 days, 1072 men (38.4%) and 553 women (34.3%) died. Severe AKI occurred in 590 men (21.8%), and 239 women (15.5%), while respiratory failure occurred in 2255 men (80.7%) and 1234 women (76.5%). After adjusting for age, race/ethnicity and clinical variables, compared with women, men had a higher risk of death (OR, 1.50, 95% CI, 1.26–1.77), severe AKI (OR, 1.92; 95% CI 1.57–2.36), and respiratory failure (OR, 1.42; 95% CI, 1.11–1.80).
In this multicenter cohort of critically ill adults with COVID-19, men were more likely to have adverse outcomes compared with women.
1. Introduction
The prevalence of coronavirus disease 2019 (COVID-19) appears to be similar in men and women, based on sex-disaggregated national statistical data reported by over 70 countries.[1,2] However, men have a higher risk of death and other adverse outcomes compared with women.[3–7] Reasons for these differences are unclear. Proposed mechanisms include higher prevalence of cigarette smoking among men, differential expression of angiotensin-converting enzyme 2 (the receptor for severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]) between men and women, and sex differences in immune response.[8–11] However, our understanding is limited because prior studies have not provided detailed clinical characteristics stratified by sex. Recognizing the importance of observed sex differences in COVID-19 outcomes, the European Association of Science Editors and other organizations urged all involved in collecting COVID-19 data to include sex data.[2,12,13] This is particularly important because sex, along with other key variables, can influence disease progression as well as access to health care services.[14] To examine factors that may play a role in sex differences in outcomes, we evaluated mortality, severe acute kidney injury (AKI), and respiratory failure in men versus women enrolled in the Study of the Treatment and Outcomes in Critically Ill Patients with COVID-19 (STOP-COVID).
2. Methods
2.1. Study design and patient population
Details regarding the design and methods of STOP-COVID have been previously published.[15] The study enrolled consecutive adults (≥18 years old) with laboratory-confirmed COVID-19 (detected by nasopharyngeal or oropharyngeal swab) admitted to an intensive care unit (ICU) at 67 participating hospitals in the US (Supplemental Digital Content Table 1, http://links.lww.com/MD/G621). The study was approved by the Institutional Review Board (IRB) of the STOP-COVID Coordinating Center (Mass General Brigham IRB), as well as the IRB of each of the 67 participating institutions with a waiver of informed consent. The current study included patients admitted to the ICU between March 4 and May 9, 2020, and followed patients until hospital discharge, death, or June 5, 2020, whichever came first.
2.2. Data collection
Study personnel at each site collected data by manual review of electronic medical records using a standardized case report form[15] to enter data into REDCap (Research Electronic Data Capture), a secure online data collection tool.[16] Patient-level data included baseline demographics (sex [men or women], race and ethnicity), coexisting medical conditions (including diabetes mellitus, hypertension, chronic obstructive pulmonary disease [COPD], asthma, chronic kidney disease), symptoms and medications prior to hospital admission, and vital signs on ICU admission. The definition of baseline characteristics, comorbidities, treatments and outcomes is presented in Supplemental Digital Content Table 2, http://links.lww.com/MD/G621. In addition, daily data for the 14 days following ICU admission were collected on physiologic and laboratory values, pharmacologic and non-pharmacologic treatments, and organ support.
2.3. Outcomes
The primary outcome was mortality within 28 days of ICU admission. Patients who were discharged from the hospital prior to 28 days were considered to be alive (we tested the validity of this assumption in a subset of patients, described elsewhere).[15] Secondary outcomes were severe AKI and respiratory failure occurring within 14 days of ICU admission. Respiratory failure was defined as requirement for invasive mechanical ventilation (delivered via endotracheal or tracheal tube). Severe AKI was defined as new requirement for kidney replacement therapy.[17]
2.4. Statistical analysis
Baseline characteristics stratified by sex (men and women) are summarized as mean or median (IQR) for continuous variables, and frequency (proportion) for categorical variables. Chi-squared test was used to compare categorical variables, and t-test or Wilcoxon rank sum test to compare continuous variables. To evaluate the association between sex (men vs women) and death, we performed a sequential modeling procedure using multilevel logistic regression with hospital as a random effect (to account for inter-hospital variation), and sex, as well as all other covariates as fixed effects. Covariates were chosen based on prior studies.[5–7,18] The base model contained only the exposure (sex, women or men). Model 2 additionally adjusted for age and race/ethnicity; model 3 added obesity (body mass index [BMI] ≥30 kg/m2), smoking, hypertension, diabetes mellitus, chronic kidney disease, coronary artery disease, congestive heart failure, COPD, and duration of symptoms prior to ICU admission; model 4 added baseline (at the time of ICU admission) measurements of D-dimer, ratio of the partial pressure of arterial oxygen over the fraction of inspired oxygen, lymphocyte count, the renal component of the sequential organ failure assessment score, and number of pre-COVID-19 ICU beds; model 5 added angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker, nonsteroidal anti-inflammatory drug, aspirin, and vitamin D use prior to hospital admission. We performed similar analyses for the secondary outcomes, severe AKI and respiratory failure. In analyses where AKI was the outcome, we excluded 156 patients (3.5%) with a history of end-stage kidney disease (85 men [3.0%] and 71 [4.4%] women). In addition, for the primary outcome, we conducted pre-specified stratified analyses by age (<60 or ≥ 60 years), race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, Asian/Other or unknown), hypertension, diabetes mellitus, and obesity. Formal test for interaction was performed by including an interaction term between sex and the potential effect modifier in the final regression model. As a sensitivity analysis, given that death is a competing risk for AKI, we examined the composite end point of severe AKI or death within 14 days following ICU admission.[19] Missing data were not imputed. Instead, we created a separate missing category for each covariate that had missing data (Table 1), since data may not have been missing at random.[17,20] As a sensitivity analysis, we conducted Cox proportional hazards regression analysis to evaluate the association between ethnicity and the primary outcome. All tests were 2-sided, and P < .05 was considered statistically significant for hypothesis testing. Analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Table 1.
Characteristics of adults with coronavirus disease 2019 admitted to the intensive care unit.
| Patients, No. (%) or median (IQR) | |||
| Characteristic | All (N = 4407) | Men (n = 2793) | Women (n = 1614) |
| Demographics | |||
| Age, yr | 62 (52–71) | 61 (51–70) | 64 (54–72)a |
| Race/ethnicity | |||
| Non-hispanic White | 1159 (26.3) | 773 (27.7) | 386 (23.9)a |
| Non-hispanic Black | 1220 (27.7) | 684 (24.5) | 536 (33.2) |
| Hispanic | 994 (22.6) | 689 (24.7) | 305 (18.9) |
| Asian/Other race/ethnicity | 288 (6.5) | 189 (6.8) | 99 (6.1) |
| Unknown | 746 (16.9) | 458 (16.4) | 288 (17.8) |
| Current or former smoker | 1299 (29.5) | 877 (31.4) | 422 (26.2) |
| Body mass index, kg/m2 | |||
| <30 | 1998 (45.3) | 1391 (49.8) | 607 (37.6)a |
| ≥30 | 2199 (49.9) | 1267 (45.4) | 932 (57.7) |
| Missing | 210 (4.8) | 135 (4.8) | 75 (4.7) |
| Symptoms | |||
| Cough | 3214 (72.9) | 2048 (73.3) | 1166 (72.2) |
| Sputum production | 459 (10.4) | 293 (10.5) | 166 (10.3) |
| Sore throat | 343 (7.8) | 216 (7.7) | 127 (7.9) |
| Nasal congestion | 257 (5.8) | 159 (5.7) | 98 (6.1) |
| Headache | 390 (8.9) | 234 (8.4) | 156 (9.7) |
| Fever | 2958 (67.1) | 1924 (68.9) | 1034 (64.1)a |
| Chills | 843 (19.1) | 548 (19.6) | 295 (18.3) |
| Fatigue or malaise | 1406 (31.9) | 877 (31.4) | 529 (32.8) |
| Dyspnea | 3290 (74.7) | 2092 (74.9) | 1198 (74.2) |
| Nausea or vomiting | 698 (15.8) | 386 (13.8) | 312 (19.3)a |
| Diarrhea | 898 (20.4) | 549 (19.7) | 349 (21.6) |
| Myalgia or arthralgia | 954 (21.7) | 622 (22.3) | 332 (20.6) |
| Symptom duration prior to ICU admission | 7.87 (5.8) | 8.12 (5.9) | 7.43 (5.70) |
| Comorbidities | |||
| Diabetes | 1822 (41.3) | 1079 (38.6) | 743 (46.0)a |
| Hypertension | 2711 (61.5) | 1660 (59.4) | 1051 (65.1)a |
| Chronic obstructive pulmonary disease | 373 (8.5) | 214 (7.7) | 159 (9.9)a |
| Asthma | 466 (10.6) | 196 (7.0) | 270 (16.7)a |
| Coronary artery disease | 589 (13.4) | 400 (14.3) | 189 (11.7)a |
| Congestive heart failure | 433 (9.8) | 247 (8.8) | 186 (11.5)a |
| Chronic kidney disease | 569 (12.9) | 352 (12.6) | 217 (13.4) |
| End-stage kidney disease | 156 (3.5) | 85 (3.0) | 71 (4.4)a |
| Active malignancy | 201 (4.6) | 132 (4.7) | 69 (4.3) |
| Home medications | |||
| Angiotensin-converting enzyme inhibitor | 804 (18.2) | 537 (19.2) | 267 (16.5)a |
| Angiotensin receptor blocker | 671 (15.2) | 394 (14.1) | 277 (17.2)a |
| Non-steroidal anti-inflammatory drug | 358 (8.1) | 200 (7.2) | 158 (9.8)a |
| Aspirin | 984 (22.3) | 656 (23.5) | 328 (20.3)a |
| Vitamin D | 474 (10.8) | 248 (8.9) | 226 (14.0)a |
| Vital signs on the day of ICU admission | |||
| Temperature, °C | 37.9 (37.2–38.8) | 38.1 (37.2–38.9) | 37.8 (37.2–38.6) |
| Systolic blood pressure, mm Hg | 97 (85–110) | 97 (85–111) | 96 (85–110) |
| Heart rate, /min | 105 (91–120) | 105 (91–120) | 103 (90–119) |
| Laboratory findings on the day of ICU admissionb | |||
| White blood cell count, k/μL | 8.4 (6.0–11.8) | 8.6 (6.0–11.9) | 8.2 (5.9–11.6) |
| Lymphocyte count, /μL | |||
| < 1000 | 2311 (52.4) | 1547 (55.4) | 764 (47.3) |
| ≥ 1000 | 1263 (28.7) | 728 (26.1) | 535 (33.2) |
| Missing | 833 (18.9) | 518 (18.6) | 315 (19.52) |
| Hemoglobin level, g/Dl | 12.6 (11.1–14.0) | 13.1 (11.6–14.4) | 11.80 (10.3–13.0) |
| Serum Creatinine, mg/Dl | 1.07 (0.80–1.66) | 1.14 (0.88–1.74) | 0.90 (0.70,1.43) |
| D-dimer, ng/Ml | |||
| <1000 | 965 (21.9) | 608 (21.8) | 357 (22.1) |
| 1000-2500 | 700 (15.9) | 432 (15.5) | 268 (16.6) |
| >2500 | 719 (16.3) | 482 (17.3) | 237 (14.7) |
| Missing | 2023 (45.9) | 1271 (45.5) | 752 (46.6) |
| C-reactive protein, mg/L | 152 (83–236) | 157 (87–239) | 144.0 (81–224) |
| Medications and supportive treatment for COVID-19 | |||
| Remdesivir | 278 (6.3) | 160 (5.7) | 118 (7.3)a |
| Corticosteroids | 1666 (37.8) | 1039 (37.2) | 627 (38.9) |
| Tocilizumab | 788 (17.9) | 551 (19.7) | 237 (14.7)a |
| Convalescent plasma | 134 (3.0) | 94 (3.4) | 40 (2.5) |
| Anticoagulation | 2011 (45.9) | 1328 (47.8) | 683 (42.6)a |
| Enrolled in a clinical trial | 728 (16.6) | 478 (17.2) | 250 (15.6) |
| Severity of illness on the day of ICU admission | |||
| Invasive mechanical ventilation | 2757 (62.8) | 1782 (64.1) | 975 (60.7) |
| FiO2 | 80 (60–100) | 80 (60–100) | 80 (60–100) |
| PEEP, cm H2O | 12 (10–15) | 12 (10–15) | 12 (10–15) |
| Non-invasive mechanical ventilation | 106 (2.4) | 62 (2.2) | 44 (2.7) |
| High-flow nasal cannula or nonrebreather mask | 978 (22.3) | 589 (21.2) | 389 (24.2) |
| PaO2:FiO2, mm Hg | |||
| Not mechanically ventilated | 1630 (37) | 999 (36) | 631 (39) |
| >200 | 528 (120) | 339 (12.1) | 189 (11.7) |
| 100-199 | 938 (21.3) | 608 (21.8) | 330 (20.5) |
| <100 | 814 (18.5) | 536 (19.2) | 278 (17.2) |
| Missing | 497 (11.3) | 311 (11.1) | 186 (11.5) |
| Vasopressor use | 1803 (62.4) | 1166 (63.5) | 637 (60.6) |
| Hospital size (pre-COVID ICU beds) | |||
| Small (<50) | 1520 (34.5) | 958 (34.3) | 562 (34.8) |
| Medium (50–99) | 1256 (28.5) | 805 (28.8) | 451 (27.9) |
| Large (≥100) | 1631 (37.0) | 1030 (36.9) | 601 (37.2) |
P < .05.
Data regarding hemoglobin level were missing for 251 patients (5.7%); serum creatinine for 216 (4.9%), C-reactive protein for 1650 (37.4%); anticoagulation, 29 (0.7%); and enrollment in a clinical trial, 26 (0.6%).
ICU = intensive care unit, IQR = interquartile range, PaO2:FIO2 = ratio of PaO2 over the fraction of inspired oxygen (assessed only in patients receiving invasive mechanical ventilation), PEEP = positive end-expiratory pressure.
SI conversion factors: to convert white blood cells and lymphocytes to ×109/L, multiply by 0.001; hemoglobin to grams per liter, multiply by 10; creatinine to micromoles per liter, multiply by 88.4; D-dimer to nanomoles per liter, multiply by 5.476; and C-reactive protein to milligrams per liter, multiply by 10.
3. Results
3.1. Baseline characteristics by sex
STOP-COVID enrolled 4407 participants (2793 [63%] men and 1614 [37%] women) from 67 US medical centers. Baseline characteristics, overall and stratified by sex, are shown in Table 1. The median [IQR] age was 62 [52–71] years, 1159 patients (26.3%) were non-Hispanic White, 1220 (27.7%) non-Hispanic Black, 994 (22.6%) Hispanic, and 288 (6.5%) were Asian or of another race/ethnicity. Compared with women, men were more likely to be younger (median [IQR] age 61 [51–70] vs 64 [54–72] years), Hispanic (689 [24.7%] vs 305 [18.9%]) or non-Hispanic White (773 [27.7%] vs 386 [23.9%]), and less likely to be non-Hispanic Black (684 [24.5%] vs 536 [33.2%]). Men were more likely to be current or former smokers than women (877 [31.4%] vs 422 [26.2%]). The presence of COVID-19 symptoms was similar in men and women except for fever, which was more common in men (1924 [69%] vs 1034 [64%]), and nausea or vomiting, which were less prevalent among men (386 [13.8%] vs 312 [19%]). Compared with women, men were less likely to have BMI ≥30 kg/m2 (1267 [45.4%] vs. 932 [57.7%]). The prevalence of most major comorbid conditions was lower in men than women (Table 1). There were also sex differences in renin-angiotensin system blocker use prior to hospital admission: men were more likely to use angiotensin-converting enzyme inhibitor I (537 [19.2%] vs 267 [16.5%]), and were less likely to use angiotensin II receptor blocker (394 [14.1%] vs 277 [17.2%]). Laboratory findings, critical care parameters and treatment received are presented in Table 1.
3.2. Primary outcome
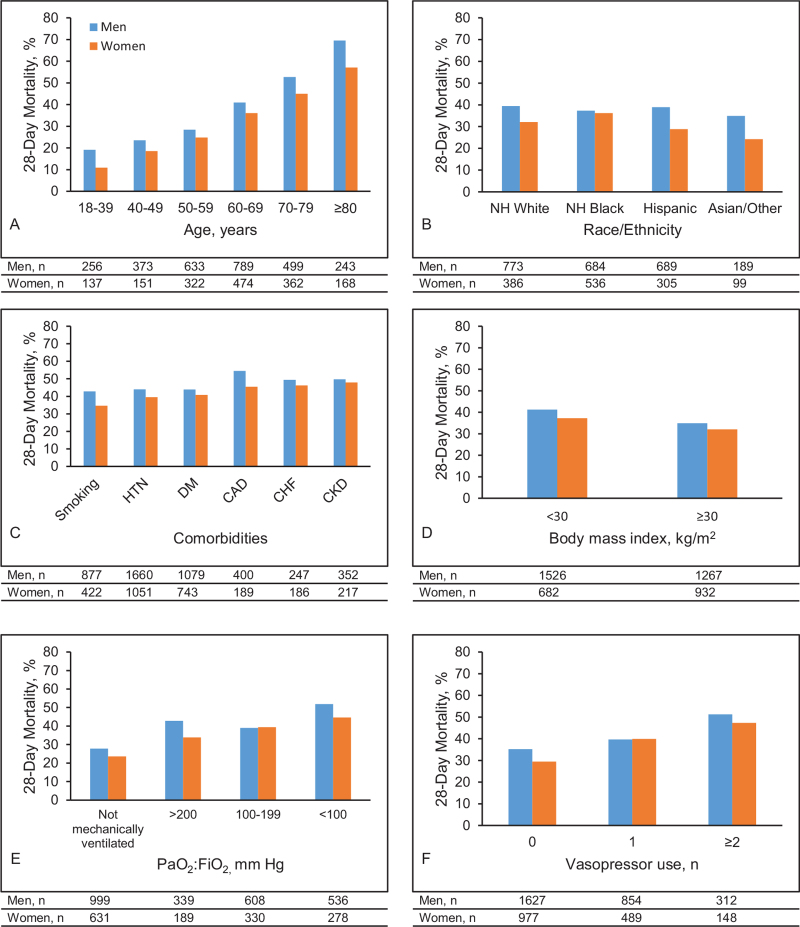
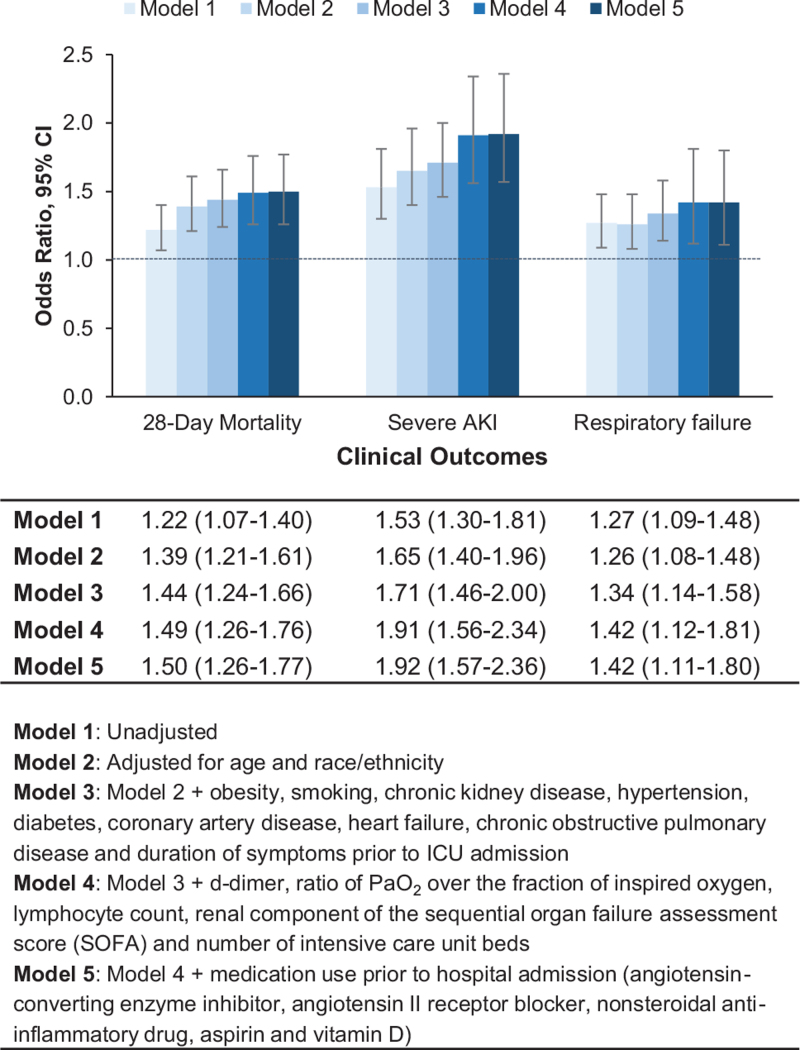
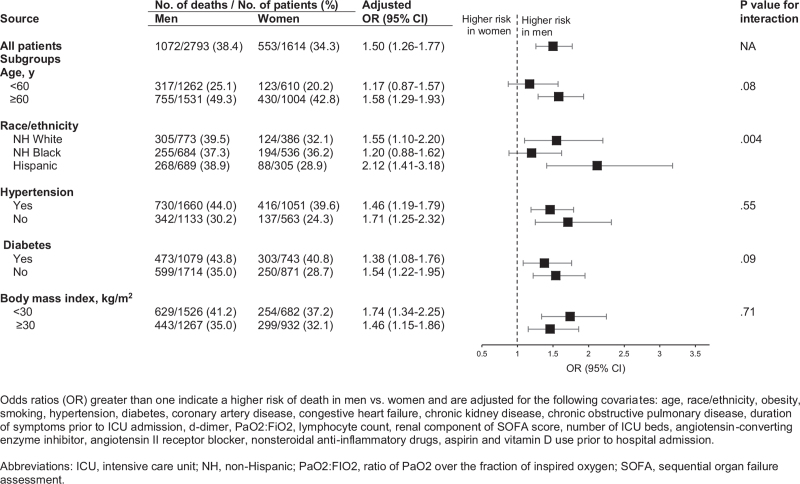
During a median (IQR) follow-up of 14 (7–24) days, 1072 men (38.4%) and 553 women (34.3%) died (Table 2). In both men and women, the most common causes of death were respiratory failure and septic shock. Death rates were higher in men than women across most subgroups based on age, race/ethnicity, comorbid conditions, BMI, and disease severity (ratio of PaO2 over the fraction of inspired oxygen and number of vasopressor medications used) (Fig. 1). The sex difference in death rates was less pronounced among non-Hispanic Black patients (men, 37.3% vs women, 36.2%) due to higher death rates in non-Hispanic Black women (36.2%) compared with non-Hispanic white women (32.2%), and Hispanic women (28.9%) (Fig. 1). In models adjusted for age and race/ethnicity (Model 2), men had a 39% higher risk of death (odds ratio [OR], 1.39; 95% confidence interval [CI], 1.21–1.61) compared with women (Fig. 2). This association remained significant and was actually strengthened after adjusting for comorbidities, laboratory values, severity of illness, hospital size and medications (Model 5, OR, 1.50; 95% CI, 1.26–1.77). Similar results were observed in analyses stratified by age, race/ethnicity, hypertension, diabetes, and obesity (Fig. 3). We found evidence of interaction between sex and race/ethnicity (P = .004). In stratified analysis, there was higher mortality risk among non-Hispanic Black males compared with non-Hispanic Black women, but it was attenuated compared with other racial/ethnic groups. The number of patients in other racial/ethnic groups was too small to conduct stratified analyses. Cox proportional regression analyses yielded similar results (multivariable-adjusted HR 1.20, 95% CI 1.07–1.36).
Table 2.
Clinical outcomes by sexa.
| Patients, No. (%) | |||
| Outcome | All (N = 4407) | Men (n = 2793) | Women (n = 1614) |
| Died within 28 d | 1625 (36.9) | 1072 (38.4) | 553 (34.3) |
| Causes of deathb | |||
| Respiratory failure | 1605 (98.8) | 1052 (98.1) | 553 (100) |
| Heart failure | 181 (11.1) | 122 (11.5) | 59 (10.7) |
| Septic shock | 710 (43.7) | 477 (44.5) | 233 (42.1) |
| Kidney failure | 619 (38.1) | 436 (40.7) | 183 (33.1) |
| Liver failure | 83 (5.1) | 51 (4.8) | 32 (5.8) |
| Other | 252 (15.5) | 178 (16.6) | 74 (13.4) |
| Severe acute kidney injuryc | 829 (19.5) | 590 (21.8) | 239 (15.5) |
| Length of kidney replacement therapy d, median (IQR) | 6 (3–10) | 6 (3–10) | 7 (3–11) |
| Respiratory failure | 3489 (79.2) | 2255 (80.7) | 1234 (76.5) |
| Length of mechanical ventilation d, median (IQR) | 11 (6–14) | 11 (6–14) | 11 (6–14) |
IQR = interquartile range.
Data are presented as number of patients (percentage).
Defined as per medical record review. Patients could have had more than one cause of death.
Patients with end-stage kidney disease (n = 156) were excluded from the denominator for analyses of acute kidney injury.
Figure 1.
Mortality by sex and pre-specified subgroups. Twenty-eight-day mortality by sex and (A) age group, (B) race/ethnicity, (C) comorbities, (D) body mass index, (E) PaO2:FIO2, and (F) vasopressor use. CAD = coronary artery disease, CHF = congestive heart failure, CKD = chronic kidney disease, DM = diabetes, HTN = hypertension, PaO2:FIO2 = ratio of PaO2 over the fraction of inspired oxygen (assessed only in patients receiving invasive mechanical ventilation).
Figure 2.
Multivariable-adjusted risk models. Odds ratio with 95% confidence interval for 28-day mortality, severe acute kidney injury and respiratory failure.
Figure 3.
Mortality in men vs women, overall and by subgroups. Mortality rates and multivariable-adjusted odds ratio with 95% confidence intervals by sex, overall and stratified by age, race/ethnicity, hypertension, diabetes, and body mass index.
3.3. Secondary outcomes
During the initial 14 days after ICU admission, 829 patients (19.5%) developed severe AKI (590 men [21.8%] and 239 women [15.5%]), while 3489 patients (79.2%) developed respiratory failure (2255 men [80.7%] and 1234 women [76.5%]) (Table 2). In multivariable analyses, men were at higher risk of developing severe AKI (OR, 1.92; 95% CI 1.57–2.36) and respiratory failure (OR, 1.42; 95% CI, 1.11–1.80) (Fig. 2). Similar results were observed when the composite outcome of severe AKI or 14-day mortality was examined (OR, 1.65; 95% CI, 1.40–1.94).
4. Discussion
This study examined sex-related differences in sociodemographic characteristics and clinical outcomes in a large, multicenter US cohort of critically ill adult men women with COVID-19. Despite being younger and having a lower burden of comorbidities compared with women, men were at significantly higher risk of death, respiratory failure and severe acute kidney injury. These findings remained robust despite extensive adjustment for age, race/ethnicity and clinical variables. Notably, the higher risk of adverse outcomes observed in men compared with women was consistent among patients with and without diabetes, hypertension or obesity.
The underlying reasons for sex-related differences in COVID-19 outcomes observed in this study population are not well understood, but are likely due to multiple factors. Although the true prevalence of COVID-19 is not known, based on national statistical data, the number of cases among men and women appears to be similar.[1,2] Therefore, worse outcomes among men might reflect underlying sex differences in biological factors or exposures that might increase the risk of severe illness. In this cohort, compared with women, men were more likely to smoke cigarettes which is a risk factor for severe COVID-19.[21] In contrast, they were younger and less likely to have a diagnosis of major comorbidities such as obesity, diabetes, hypertension, COPD, asthma and heart failure. This is likely the reason for the negative confounding observed in regression analyses, given that older age and the presence of comorbid conditions have been associated with worse COVID-19 related outcomes.[22] In addition, it is possible that compared with women, men included in our study had less adequate control of hypertension, diabetes and other cardiovascular risk factors, as has been observed in general population studies;[23,24] unfortunately, data regarding the severity and control of these conditions was not available in our study. While we were unable to confirm occupational hazards, a prior study suggested that men are more likely to hold jobs associated with increased risk of viral load exposure such as food processing, transportation and delivery.[25] It is also possible that immune system differences may contribute to the sex-related discrepancies in COVID-19 outcomes.[26] A recent study focused on patients with moderate disease reported that men had higher plasma levels of innate immune cytokines such as IL-8 and IL-18, whereas women mounted a more robust T cell activation,[11] suggesting the need to evaluate sex-based treatment approaches to the management of COVID-19. Another study focused on patients with severe COVID-19 pneumonia found that men were more likely than women to have neutralizing auto-antibodies to type I interferons, which are known to impede the ability of type I interferons to block SARS-CoV-2 infection.[27] These findings support the notion that men and women have inherent differences in their immunologic response to COVID-19 infection that may increase their risk for severe disease in a sex-dependent manner.
Our findings are consistent with prior studies reporting higher risk of death among men.[3,5,6,28,29] In a cohort study of over 17 million individuals in England, the risk of death among men was 1.6 higher than women.[6] In contrast, in a US integrated-delivery health system study of over 1300 patients hospitalized for COVID-19, the difference in mortality risk between men and women was explained by indicators of baseline vital signs and laboratory measures.[7] These heterogeneous findings could be due to differences in the populations studied as well as the severity of COVID-19 illness, with our study focusing on patients admitted to intensive care units.
Although evidence suggest that Blacks are disproportionately affected by COVID-19,[7,30,31] there has been limited exploration of sex differences in outcomes among Blacks. In a recent study of 3481 patients with COVID-19, Black race was associated with increased risk for hospitalization but not mortality.[7] However, these analyses were not stratified by sex. We found that among Blacks, the risk of death was higher in men vs. women, but this difference is attenuated compared with other racial/ethnic groups. Furthermore, we found higher rates of death among Black women compared to other women, indicating that Black women are at particularly high risk. Future research is needed to better understand reasons for these findings.
In addition to differences in mortality, we observed increased risk of severe acute kidney injury and respiratory failure in men compared with women. Our findings are consistent with a study by Fisher et al, which compared AKI outcomes in patients with and without COVID-19 hospitalized in a large New York City health system. The investigators found that male sex was associated with a higher risk of kidney replacement therapy or death regardless of COVID-19 status.[32] Similarly, a recent meta-analysis of 28 non-COVID-19 population studies reported a two-fold higher risk of severe AKI among men.[33] Based on experimental data, Neugarten et al have proposed that this female reno-protection is mediated by effects of sex hormones on cellular processes which are important in the pathogenesis of AKI.[33–35] Women in our cohort were predominantly in the post-menopausal age range, therefore the protective role of sex hormones may be attenuated. Data regarding sex-differences in respiratory failure in the context of COVID-19 are more scarce. In a retrospective study of 130 adult patients in China, the rate of acute respiratory distress syndrome (ARDS) was similar in men and women.[36] However, in a large study of over 10 thousand patients who tested positive for SARS-CoV-2 in the Veterans Affairs health care system, male sex was associated with an almost 3-fold higher risk of requiring mechanical ventilation.[29] Traditionally, male sex has not been considered an independent risk factor for the development of ARDS in general populations. For example, the Lung Injury Prediction Score Study found no significant association.[37] However, multiple studies have reported higher number of male than female patients with ARDS, suggesting an association with other risk factors or comorbidities that are more prevalent in men.[38,39]
Findings from this study should be interpreted in light of its strengths and limitations. Some of its strengths include the comprehensive medical record data collection from a large number of critically ill patients with laboratory-confirmed COVID-19 treated at 67 centers across the US Although in-hospital mortality was ascertained over a period of 28 days, data regarding severe AKI and respiratory failure were only collected for the first 14 days of ICU admission; therefore, the true incidence of these outcomes might have been underestimated. In addition, the study did not include individuals from other countries. Thus, the findings may not be generalizable outside of the US. Lastly, as with all observational studies there may be residual confounding by unmeasured variables. For example, our analyses were not able to account for differences in immune system response or the role of sex hormones.
In this US multicenter cohort of critically ill adults with COVID-19, men were more likely to have adverse outcomes compared with women, even after accounting for the higher burden of chronic disease among women. Future therapeutic studies are needed to focus on sex differences in the immune system response to SARS-CoV-2 and the potential role of sex hormones in outcomes.
Stop-Covid Investigators
Baylor College of Medicine: Carl P. Walther∗, Samaya J. Anumudu.
Baylor University Medical Center: Justin Arunthamakun∗, Kathleen F. Kopecky, Gregory P. Milligan, Peter A. McCullough, Thuy-Duyen Nguyen.
Beth Israel Deaconess Medical Center: Shahzad Shaefi∗, Megan L. Krajewski, Sidharth Shankar, Ameeka Pannu, Juan D. Valencia.
Boston Medical Center: Sushrut S. Waikar∗, Zoe A. Kibbelaar.
Cook County Health: Ambarish M. Athavale∗, Peter Hart, Shristi Upadhyay, Ishaan Vohra, Ajiboye Oyintayo.
Cooper University Health Care: Adam Green∗, Jean-Sebastien Rachoin, Christa A. Schorr, Lisa Shea.
Duke University Medical Center: Daniel L. Edmonston∗, Christopher L. Mosher.
Hackensack Meridian Health Mountainside Medical Center: Alexandre M. Shehata∗, Zaza Cohen, Valerie Allusson, Gabriela Bambrick-Santoyo, Noor ul aain Bhatti, Bijal Mehta, Aquino Williams.
Hackensack Meridian Health Hackensack University Medical Center: Samantha K. Brenner∗, Patricia Walters, Ronaldo C. Go, Keith M. Rose.
Harvard T.H. Chan School of Public Health: Miguel A. Hernán.
Harvard University: Amy M. Zhou, Ethan C. Kim, Rebecca Lisk.
Icahn School of Medicine at Mount Sinai: Lili Chan∗, Kusum S. Mathews∗, Steven G. Coca, Deena R. Altman, Aparna Saha, Howard Soh, Huei Hsun Wen, Sonali Bose, Emily A. Leven, Jing G. Wang, Gohar Mosoyan, Girish N. Nadkarni, Pattharawin Pattharanitima, Emily J. Gallagher.
Indiana University School of Medicine/Indiana University Health: Allon N. Friedman∗, John Guirguis, Rajat Kapoor, Christopher Meshberger, Katherine J. Kelly.
Johns Hopkins Hospital: Chirag R. Parikh∗, Brian T. Garibaldi, Celia P. Corona-Villalobos, Yumeng Wen, Steven Menez, Rubab F. Malik, Carmen Elena Cervantes, Samir C. Gautam.
Kings County Hospital Center: Mary C. Mallappallil∗, Jie Ouyang, Sabu John, Ernie Yap, Yohannes Melaku, Ibrahim Mohamed, Siddhartha Bajracharya, Isha Puri, Mariah Thaxton, Jyotsna Bhattacharya, John Wagner, Leon Boudourakis.
Loma Linda University: H. Bryant Nguyen∗, Afshin Ahoubim.
Mayo Clinic, Arizona: Leslie F. Thomas∗, Dheeraj Reddy Sirganagari.
Mayo Clinic, Florida: Pramod K. Guru∗.
Mayo Clinic, Rochester: Kianoush Kashani∗, Shahrzad Tehranian.
Medical College of Wisconsin: Yan Zhou,∗ Paul A. Bergl, Jesus Rodriguez, Jatan A. Shah, Mrigank S. Gupta.
MedStar Georgetown University Hospital: Princy N. Kumar∗, Deepa G. Lazarous, Seble G. Kassaye.
Montefiore Medical Center/Albert Einstein College of Medicine: Michal L. Melamed∗, Tanya S. Johns, Ryan Mocerino, Kalyan Prudhvi, Denzel Zhu, Rebecca V. Levy, Yorg Azzi, Molly Fisher, Milagros Yunes, Kaltrina Sedaliu, Ladan Golestaneh, Maureen Brogan, Neelja Kumar, Michael Chang, Jyotsana Thakkar.
New York-Presbyterian Queens Hospital: Ritesh Raichoudhury∗, Akshay Athreya, Mohamed Farag.
New York-Presbyterian/Weill Cornell Medical Center: Edward J. Schenck∗, Soo Jung Cho, Maria Plataki, Sergio L. Alvarez-Mulett, Luis G. Gomez-Escobar, Di Pan, Stefi Lee, Jamuna Krishnan, William Whalen.
New York University Langone Hospital: David Charytan∗, Ashley Macina, Sobaata Chaudhry, Benjamin Wu, Frank Modersitzki.
Northwestern Memorial Hospital: Northwestern University Feinberg School of Medicine - Anand Srivastava∗, Alexander S. Leidner, Carlos Martinez, Jacqueline M. Kruser, Richard G. Wunderink, Alexander J. Hodakowski.
Ochsner Medical Center: Juan Carlos Q. Velez∗, Eboni G. Price-Haywood, Luis A. Matute-Trochez, Anna E. Hasty, Muner MB. Mohamed.
Oregon Health and Science University Hospital: Rupali S. Avasare∗, David Zonies∗.
Partners Healthcare: Brigham and Women's Hospital, Brigham and Women's Faulkner Hospital, Massachusetts General Hospital, and Newton Wellesley Hospital - David E. Leaf∗, Shruti Gupta∗, Meghan E. Sise, Erik T. Newman, Samah Abu Omar, Kapil K. Pokharel, Shreyak Sharma, Harkarandeep Singh, Simon Correa, Tanveer Shaukat, Omer Kamal, Wei Wang, Heather Yang, Jeffery O. Boateng, Meghan Lee, Ian A. Strohbehn, Jiahua Li, Ariel L. Mueller.
ProMedica Health System: Roberta E. Redfern,∗ Nicholas S. Cairl, Gabriel Naimy, Abeer Abu-Saif, Danyell Hall, Laura Bickley.
Renown Health: Chris Rowan∗, Farah Madhani-Lovely∗, Vivian S. Cruz, Kristen M. Hess, Alanna L. Jacobs.
Rush University Medical Center: Vasil Peev∗, Jochen Reiser, John J. Byun, Andrew Vissing, Esha M. Kapania, Zoe Post, Nilam P. Patel, Joy-Marie Hermes.
Rutgers/New Jersey Medical School: Anne K. Sutherland∗, Amee Patrawalla, Diana G. Finkel, Barbara A. Danek, Sowminya Arikapudi, Jeffrey M. Paer, Peter Cangialosi, Mark Liotta.
Rutgers/Robert Wood Johnson Medical School: Jared Radbel∗, Sonika Puri, Jag Sunderram, Matthew T. Scharf, Ayesha Ahmed, Ilya Berim, Jayanth S. Vatson.
Stanford Healthcare: Stanford University School of Medicine – Shuchi Anand∗, Joseph E. Levitt, Pablo Garcia.
Temple University Hospital: Suzanne M. Boyle∗, Rui Song.
Thomas Jefferson University Hospital: Jingjing Zhang∗, Sang Hoon Woo, Xiaoying Deng, Goni Katz-Greenberg, Katharine Senter.
Tulane Medical Center: Moh’d A. Sharshir∗, Vadym V. Rusnak.
United Health Services Hospitals: Muhammad Imran Ali.
University of Colorado Anschutz Medical Campus: Anip Bansal∗, Amber S. Podoll, Michel Chonchol, Sunita Sharma, Ellen L. Burnham, David J. Douin.
University Hospitals Cleveland Medical Center: Arash Rashidi∗, Rana Hejal.
University of Alabama-Birmingham Hospital: Eric Judd∗, Laura Latta, Ashita Tolwani.
University of California-Davis Medical Center: Timothy E. Albertson∗, Jason Y. Adams.
University of California-Los Angeles Medical Center: Ronald Reagan-UCLA Medical Center - Steven Y. Chang∗, Rebecca M. Beutler; UCLA Medical Center, Santa Monica – Carl E. Schulze.
University of California-San Diego Medical Center: Etienne Macedo∗, Harin Rhee.
University of California-San Francisco Medical Center: Kathleen D. Liu∗, Vasantha K. Jotwani.
University of Chicago Medical Center: Jay L. Koyner∗.
University of Florida Health-Gainesville: Chintan V. Shah∗.
University of Florida-Health-Jacksonville: Vishal Jaikaransingh∗.
University of Illinois Hospital and Health Sciences System: Stephanie M. Toth-Manikowski∗, Min J. Joo∗, James P. Lash.
University of Kentucky Medical Center: Javier A. Neyra∗, Nourhan Chaaban, Madona Elias, Yahya Ahmad.
University Medical Center of Southern Nevada: Rajany Dy∗, Alfredo Iardino, Elizabeth H. Au, Jill H. Sharma.
University of Miami Health System: Marie Anne Sosa∗, Sabrina Taldone, Gabriel Contreras, David De La Zerda, Hayley B. Gershengorn, Bhavarth Shukla, Alessia Fornoni, Tanira Ferreira.
University of Michigan: Salim S. Hayek∗, Pennelope Blakely, Hanna Berlin, Tariq U. Azam, Husam Shadid, Michael Pan, Patrick O’ Hayer, Chelsea Meloche, Rafey Feroze, Rayan Kaakati, Danny Perry, Abbas Bitar, Elizabeth Anderson, Kishan J. Padalia, John P. Donnelly, Andrew J. Admon.
University of North Carolina School of Medicine: Jennifer E. Flythe∗, Matthew J. Tugman, Emily H. Chang.
University of Oklahoma Health Sciences Center: Brent R. Brown∗.
University of Pennsylvania Health System: Amanda K. Leonberg-Yoo∗, Ryan C. Spiardi, Todd A. Miano, Meaghan S. Roche, Charles R. Vasquez.
University of Pittsburgh Medical Center: Amar D. Bansal∗, Natalie C. Ernecoff, Sanjana Kapoor, Siddharth Verma, Huiwen Chen.
University of Tennessee Health Science Center and Memphis VA Medical Center/Methodist.
University Hospital – Csaba P. Kovesdy∗, Miklos Z. Molnar∗, Ambreen Azhar.
University of Texas Southwestern Medical Center and Parkland Health and Hospital System: S. Susan Hedayati∗, Mridula V. Nadamuni, Shani Shastri, Duwayne L. Willett.
University of Vermont Larner College of Medicine: Samuel A.P. Short.
University of Virginia Health System: Amanda D. Renaghan∗, Kyle B. Enfield.
University of Washington Medical Center: Pavan K. Bhatraju∗, A. Bilal Malik.
Vanderbilt University Medical Center: Matthew W. Semler.
Washington University in St. Louis/Barnes Jewish Hospital: Anitha Vijayan∗, Christina Mariyam Joy, Tingting Li, Seth Goldberg, Patricia F. Kao.
Wellforce Health System: Lowell General Hospital - Greg L. Schumaker∗, Tufts Medical Center - Nitender Goyal∗, Anthony J. Faugno, Greg L. Schumaker, Caroline M. Hsu, Asma Tariq, Leah Meyer, Ravi K. Kshirsagar, Daniel E. Weiner, Aju Jose.
Westchester Medical Center: Marta Christov∗, Jennifer Griffiths, Sanjeev Gupta, Aromma Kapoor, Savneek Chugh.
Yale School of Medicine: Perry Wilson,∗ Tanima Arora, Ugochukwu Ugwuowo.
∗Site Principal Investigator.
Author contributions
SMT-C, JPL, SG, DEL and ACR designed the study. All authors contributed to data collection and data interpretation, and revised the manuscript for important intellectual content. ACR is the guarantor of the paper, taking responsibility for the integrity of the work as a whole.
Conceptualization: Stephanie Toth-Manikowski, Shruti Gupta, James P Lash, David E Leaf, Ana C. Ricardo.
Data curation: Stephanie Toth-Manikowski, Jillian Caldwell, Min Joo, Natalie Meza, Jacob Bruinius, Shruti Gupta, Mary Hannan, Mustafa Kagalwalla, Samantha Madrid, Michal Melamed, Esther Pacheco, Anand Srivastava, Christopher Viamontes, James P Lash, David E Leaf.
Formal analysis: Jinsong Chen.
Investigation: Jillian Caldwell, Michal Melamed, Anand Srivastava, James P Lash, David E Leaf.
Methodology: Jinsong Chen, James P Lash, David E Leaf.
Project administration: Natalie Meza, Shruti Gupta, Anand Srivastava, James P Lash, David E Leaf.
Resources: Jillian Caldwell, Natalie Meza, Jacob Bruinius, Samantha Madrid, Michal Melamed, Anand Srivastava, James P Lash, David E Leaf.
Software: Jinsong Chen.
Supervision: Min Joo, Natalie Meza, Shruti Gupta, James P Lash, David E Leaf.
Writing – original draft: Jillian Caldwell, James P Lash, Ana C. Ricardo.
Writing – review & editing: Stephanie Toth-Manikowski, Jillian Caldwell, Min Joo, Jacob Bruinius, Shruti Gupta, Mary Hannan, Mustafa Kagalwalla, Samantha Madrid, Michal Melamed, Esther Pacheco, Anand Srivastava, Christopher Viamontes, James P Lash, David E Leaf, Ana C. Ricardo.
Footnotes
Abbreviations: AKI = acute kidney injury, BMI = body mass index, COPD = chronic obstructive pulmonary disease, COVID-19 = coronavirus disease 2019, ICU = intensive care unit, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2, STOP-COVID = study of the treatment and outcomes in critically Ill patients with COVID-19.
How to cite this article: Toth-Manikowski SM, Caldwell J, Joo M, Chen J, Meza N, Bruinius J, Gupta S, Hannan M, Kagalwalla M, Madrid S, Melamed ML, Pacheco E, Srivastava A, Viamontes C, Lash JP, Leaf DE, Ricardo AC. Sex-related differences in mortality, acute kidney injury, and respiratory failure among critically ill patients with COVID-19. Medicine. 2021;100:50(e28302).
DEL, ACR, SMT-M and JC contributed equally to the work.
No funding was provided for this study. The authors of the writing committee are supported by the following grants from the National Institutes of Health: R01DK118736 (A.C.R.); 01DK072231–13S1 (S.M.T-M); K24DK092290 and R01DK072231–91 (J.P.L.); R01HL144566 and R01DK125786 (D.E.L.); F32DC017342 (S.G.); K23DK120811 (A.S.); T32HL134634 (M.H.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. M.H. is a Robert Wood Johnson Foundation Future of Nursing Scholar Postdoctoral Fellow. The views expressed here do not necessarily reflect the views of the Foundation.
Disclosures: D.E.L. received research support from BioPorto. S.G. is a scientific coordinator for the ASCEND trial (GlaskoSmithKline) and receives research funding from GE Healthcare and BTG International. A.S. reports personal fees from Horizon Therapeutics, PLC, AstraZeneca, CVS Caremark, and medicolegal consulting (Tate & Latham).
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
References
- [1].COVID-19 sex-disaggregated data tracker – Global Health 50/50. Available at: https://globalhealth5050.org/covid19/sex-disaggregated-data-tracker/. Accessed November 2, 2021. [Google Scholar]
- [2].Bhopal SS, Bhopal R. Sex differential in COVID-19 mortality varies markedly by age. Lancet 2020;396:532–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA 2020;323:2052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yu C, Lei Q, Li W, et al. Clinical characteristics, associated factors, and predicting COVID-19 mortality risk: a retrospective study in Wuhan, China. Am J Prev Med 2020;59:168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ 2020;369:01–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020;584:430–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Price-Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. N Engl J Med 2020;382:2534–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gemmati D, Bramanti B, Serino ML, Secchiero P, Zauli G, Tisato V. COVID-19 and individual genetic susceptibility/receptivity: role of ACE1/ACE2 genes, immunity, inflammation and coagulation. might the double X-chromosome in females be protective against SARS-CoV-2 compared to the single X-chromosome in males? Int J Mol Sci 2020;21:01–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cai H. Sex difference and smoking predisposition in patients with COVID-19. Lancet Respir Med 2020;8:e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gagliardi MC, Tieri P, Ortona E, Ruggieri A. ACE2 expression and sex disparity in COVID-19. Cell Death Discov 2020;6:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Takahashi T, Ellingson MK, Wong P, et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature 2020;588:315–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].The Lancet. The gendered dimensions of COVID-19. Lancet 2020;395:1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. EASE Statement on Quality Standards: EASE. Accessed November 2, 2021. Available at: https://ease.org.uk/publications/ease-statements-resources/ease-statement-on-quality-standards/. [Google Scholar]
- [14].Heidari S, Ahumada C, Kurbanova Z. GENDRO Gender, Evidence and Health Network. Towards the real-time inclusion of sex- and age-disaggregated data in pandemic responses. BMJ Glob Health 2020;5:e003848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gupta S, Hayek SS, Wang W, et al. Factors associated with death in critically Ill patients with coronavirus disease 2019 in the US. JAMA Intern Med 2020;180:1436–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gupta S, Coca SG, Chan L, et al. AKI treated with renal replacement therapy in critically Ill patients with COVID-19. J Am Soc Nephrol 2021;32:161–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Albitar O, Ballouze R, Ooi JP, Sheikh Ghadzi SM. Risk factors for mortality among COVID-19 patients. Diabetes Res Clin Pract 2020;166:01–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Leaf DE, Waikar SS. End points for clinical trials in acute kidney injury. Am J Kidney Dis 2017;69:108–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gupta S, Wang W, Hayek SS, et al. Association between early treatment with tocilizumab and mortality among critically Ill patients with COVID-19. JAMA Intern Med 2021;181:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Patanavanich R, Glantz SA. Smoking Is associated with COVID-19 progression: a meta-analysis. Nicotine Tob Res 2020;22:1653–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ssentongo P, Ssentongo AE, Heilbrunn ES, Ba DM, Chinchilli VM. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: a systematic review and meta-analysis. PLoS One 2020;15:01–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ostchega Y, Zhang G, Hughes JP, Nwankwo T. Factors associated with hypertension control in US adults using 2017 ACC/AHA Guidelines: National Health and Nutrition Examination Survey 1999-2016∗∗∗. Am J Hypertens 2018;31:886–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Peters SAE, Muntner P, Woodward M. Sex differences in the prevalence of, and trends in, cardiovascular risk factors, treatment, and control in the United States, 2001 to 2016. Circulation 2019;139:1025–35. [DOI] [PubMed] [Google Scholar]
- [25].Griffith DM, Sharma G, Holliday CS, et al. Men and COVID-19: a biopsychosocial approach to understanding sex differences in mortality and recommendations for practice and policy interventions. Prev Chronic Dis 2020;17:01–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Alwani M, Yassin A, Al-Zoubi RM, et al. Sex-based differences in severity and mortality in COVID-19. Rev Med Virol 2021;31:e2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bastard P, Rosen LB, Zhang Q, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020;370:01–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Li LQ, Huang T, Wang YQ, et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol 2020;92:577–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ioannou GN, Locke E, Green P, et al. Risk factors for hospitalization, mechanical ventilation, or death among 10 131 US Veterans with SARS-CoV-2 infection. JAMA Netw Open 2020;3:01–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yehia BR, Winegar A, Fogel R, et al. Association of race with mortality among patients hospitalized with coronavirus disease 2019 (COVID-19) at 92 US hospitals. JAMA network open 2020;3:01–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].CDC. Coronavirus Disease 2019 (COVID-19) in the U.S. Centers for Disease Control and Prevention. Published March 28, 2020. Accessed November 2, 2021. Available at: https://covid.cdc.gov/covid-data-tracker. [Google Scholar]
- [32].Fisher M, Neugarten J, Bellin E, et al. AKI in hospitalized patients with and without COVID-19: a comparison study. J Am Soc Nephrol 2020;31:2145–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Neugarten J, Golestaneh L, Kolhe NV. Sex differences in acute kidney injury requiring dialysis. BMC Nephrol 2018;19:01–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Metcalfe PD, Meldrum KK. Sex differences and the role of sex steroids in renal injury. J Urol 2006;176:15–21. [DOI] [PubMed] [Google Scholar]
- [35].Hutchens MP, Dunlap J, Hurn PD, Jarnberg PO. Renal ischemia: does sex matter? Anesth Analg 2008;107:239–49. [DOI] [PubMed] [Google Scholar]
- [36].Wang A, Gao G, Wang S, et al. Clinical characteristics and risk factors of acute respiratory distress syndrome (ARDS) in COVID-19 patients in Beijing, China: a retrospective study. Med Sci Monit 2020;26:01–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Gajic O, Dabbagh O, Park PK, et al. Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. Am J Respir Crit Care Med 2011;183:462–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ely EW, Wheeler AP, Thompson BT, Ancukiewicz M, Steinberg KP, Bernard GR. Recovery rate and prognosis in older persons who develop acute lung injury and the acute respiratory distress syndrome. Ann Int Med 2002;136:25–36. [PubMed] [Google Scholar]
- [39].Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med 2005;353:1685–93. [DOI] [PubMed] [Google Scholar]