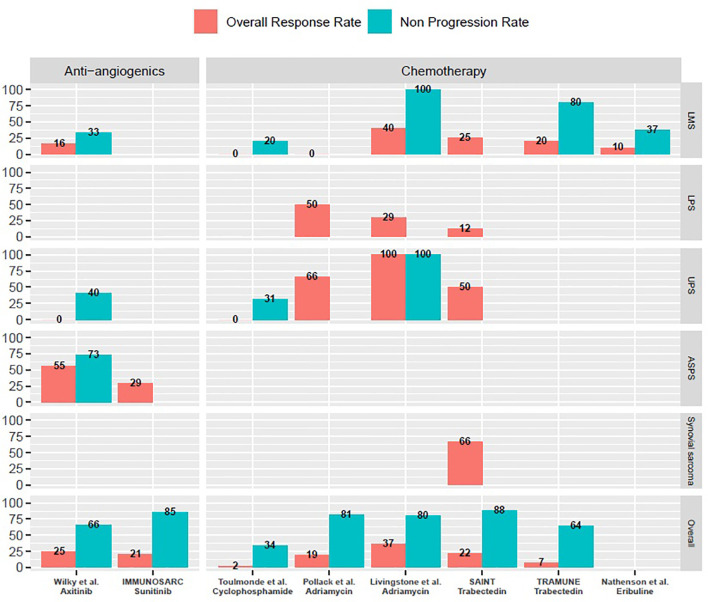
Figure 1.
Overall response rates and non-progression rates of selected prospective trials of combination treatments with immune-checkpoint inhibitors mentioned in the manuscript. ASPS, Alveolar soft-part sarcomas; LMS, Leiomyosarcoma; LPS, Liposarcoma; UPS, Undifferentiated pleomorphic sarcomas. M, months; w, weeks. Only cohorts including a minimum of three patients are reported on this graph. Non-progression rates are either three months progression-free survival rates or disease control rates at first evaluation, as reported in specific trials and mentioned throughout the manuscript.