Fig. 5. Single-molecule mechanical fingerprinting of post-translational modifications in a heterogeneous mixture of peptides.
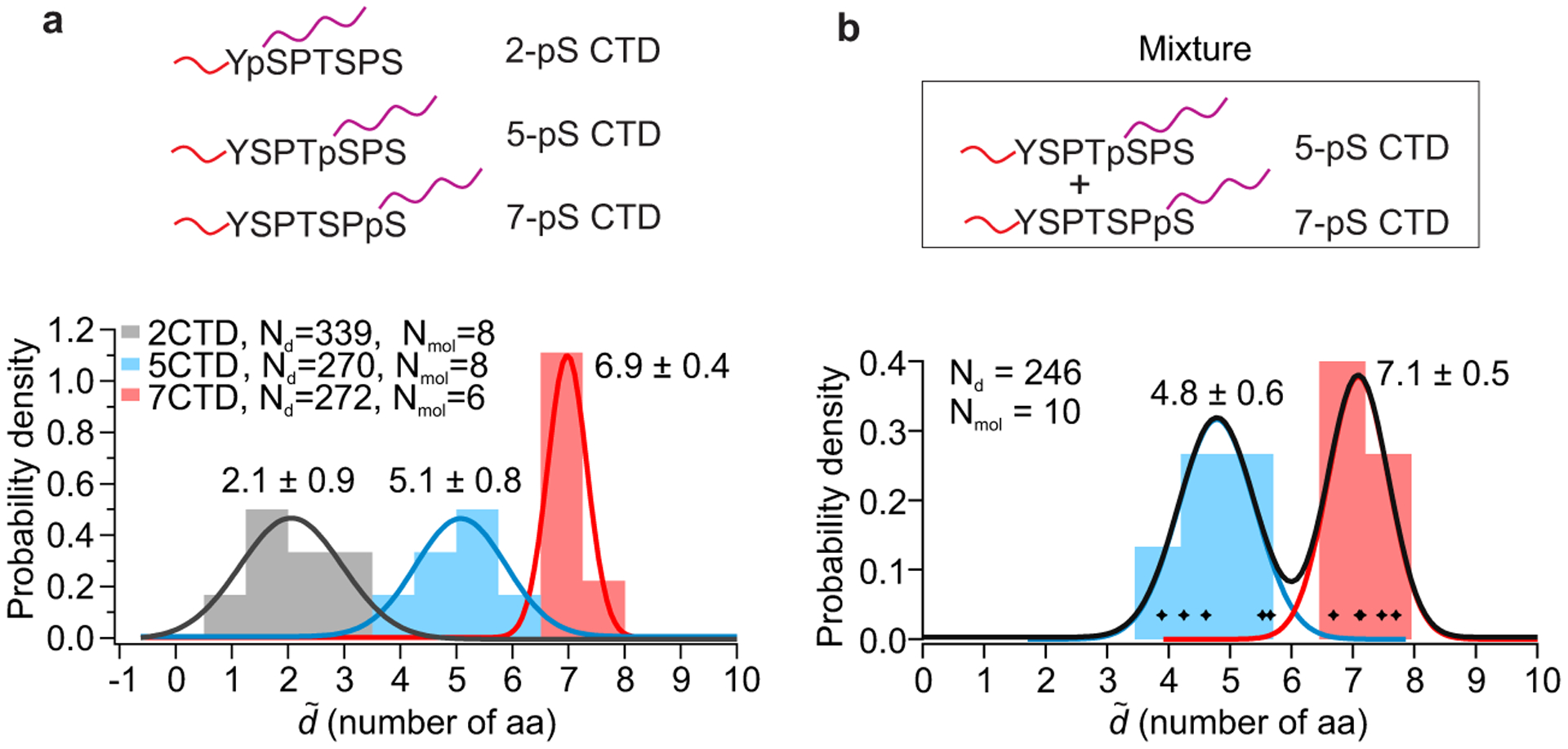
a, Characterization of post-translational modifications in C-terminal domains (CTD) of RNA Polymerase II. Long DNA handles were attached to phosphorylated serines at positions 2, 5 or 7 to measure distances from the N terminus, where a short DNA handle is attached (Top panel). Stacked histogram of the dimensionless absolute distance in units of the number of amino-acid residues between handles (, number of aa, localized per molecule) of the three different peptides is shown below, with Gaussian fits superimposed and labeled by peak value ± standard deviation. ΔL measurements presented without per molecule averaging and used for calibration can be seen in Supplementary Figures 5b&c. b, Demonstration of peptide identification in a heterogeneous sample. Histogram depicts the dimensionless absolute distance (, number of aa, localized per molecule) obtained by analyzing 10 molecules in a 1:1 mixture of 5- and 7-phosphoserine CTD peptides; molecules were classified based on the measured distance, and color-coded accordingly. Gaussian fits labeled by peak value ± standard deviation are superimposed.
