Figure 7.
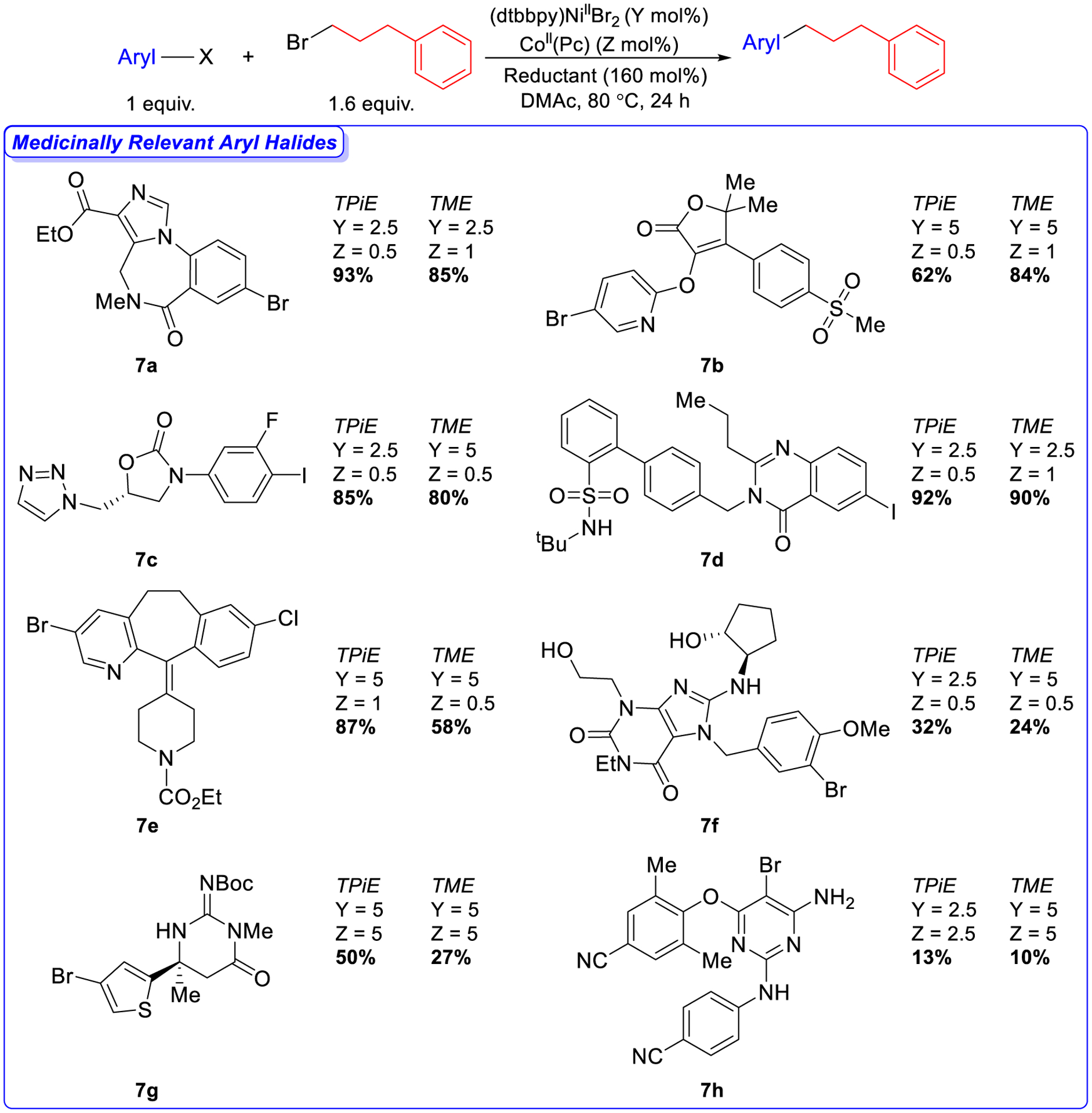
Dual-catalyzed XEC of medicinally relevant aryl halides with 1-bromo-3-phenylpropane using TPiE and TME as the reductants. Reaction conditions: aryl halides (0.0300 mmol), 1-bromo-3phenylpropane (0.0480 mmol), reductant (0.0480 mmol), (dtbbpy)NiIIBr2 (Y mol%), CoII(Pc) (Z mol%) in DMAc (0.3 mL) at 80 °C for 24 h. The yields were determined by integration of 1H NMR spectra against a hexamethylbenzene external standard.
