Table 3:
Yields of XEC reactions between 1°, 2°, and benzylic Katritzky salts and alkyl iodides with different homogeneous reductants. Reaction conditions: 2-iodoanisole (0.03125 mmol), Katritzky salt (0.0375 mmol), reductant (0.0375 mmol), (dtbbpy)NiIIBr2 (10 mol%), in DMAc (0.25 mL) at 80 °C for 24 h. The yields were determined by integration of 1H NMR spectra against a hexamethylbenzene external standard.
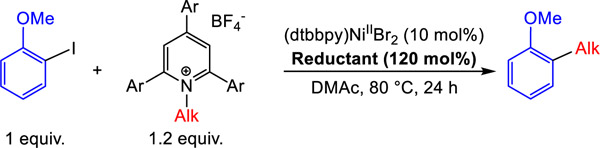
| |||||
|---|---|---|---|---|---|
| Reductant | E° (V vs Fc) | Yield (%) with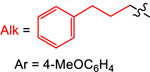
|
Yield (%) with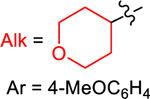
|
Yield (%) with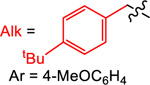
|
Yield (%) with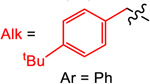
|
| 1 | 2 | 3 | 4 | ||
| TPyE | −1.32 | 77 | 34 | 57 | 25 |
| TDAE | −1.11 | 10 | 77 | 99 | 29 |
| TAzE | −1.09 | <1 | 66 | 93 | 29 |
| TPiE | −1.06 | <1 | 83 | 99 | 30 |
| TME | −0.85 | <1 | 43 | 75 (93a) | 90 |
5 mol% (dtbbpy)NiIIBr2 used.
