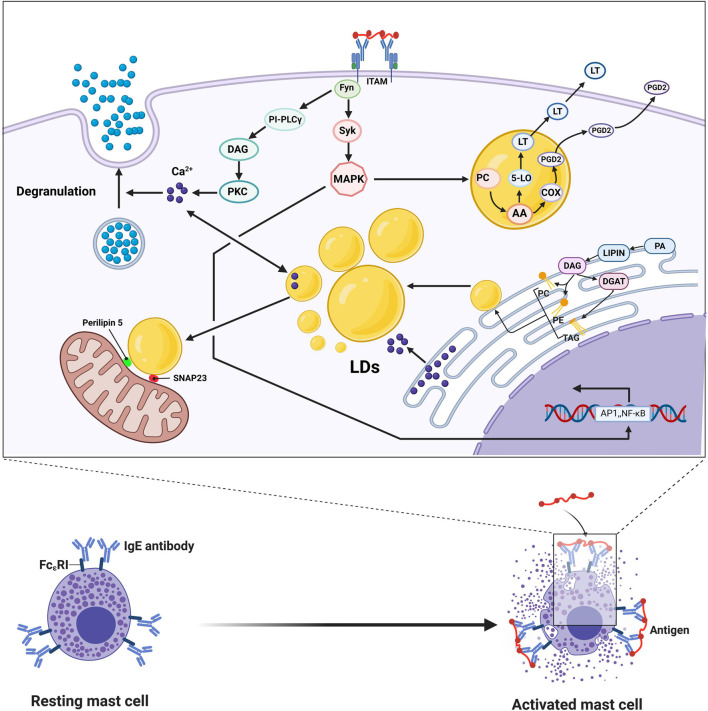
FIGURE 3.
IgE cross-linking mediated activation of mast cells leads to degranulation and release of large amounts of bioactive lipids, including prostaglandin D2 and leukotriene (LT). After FcεRI is cross-linked with each other, the phosphorylation of ITAM activates Fyn and Syk, which in turn mediates activation of PKC through the PI-PLCγ signaling chain, resulting in increased intracellular calcium concentration and phosphorylation of the intracytoplasmic myosin light chain, leading to degranulation and release of bioactive mediators. Phosphorylation of ITAM activates the mitogen-activated protein (MAK) kinase signaling pathway, causing the breakdown of phospholipid choline (PC) in the presence of phospholipase A2 to produce arachidonic acid (AA), which is then combined into PGD2 and LT, respectively, via the cyclocythase and lipoxygenase pathways. Activation of MAPK also increases the transcription of several nuclear transcription factors, such as NF-κB and AP1. After FcεRI is cross-linked, LDs are not only the source of calcium ions in mast cells but also the sinks of calcium ions, acting as the source and absorber of calcium ions, thereby affecting the corresponding signal pathways. The LDs-associated proteins Perilipin 5 and SNAP23 play crucial roles in the physical and metabolic connections between LDs and mitochondria. The synthesis of LDs is mainly carried out in the endoplasmic reticulum, accompanied by the synthesis of TAG, PC, and PE with FA as the substrate under the action of various enzymes.