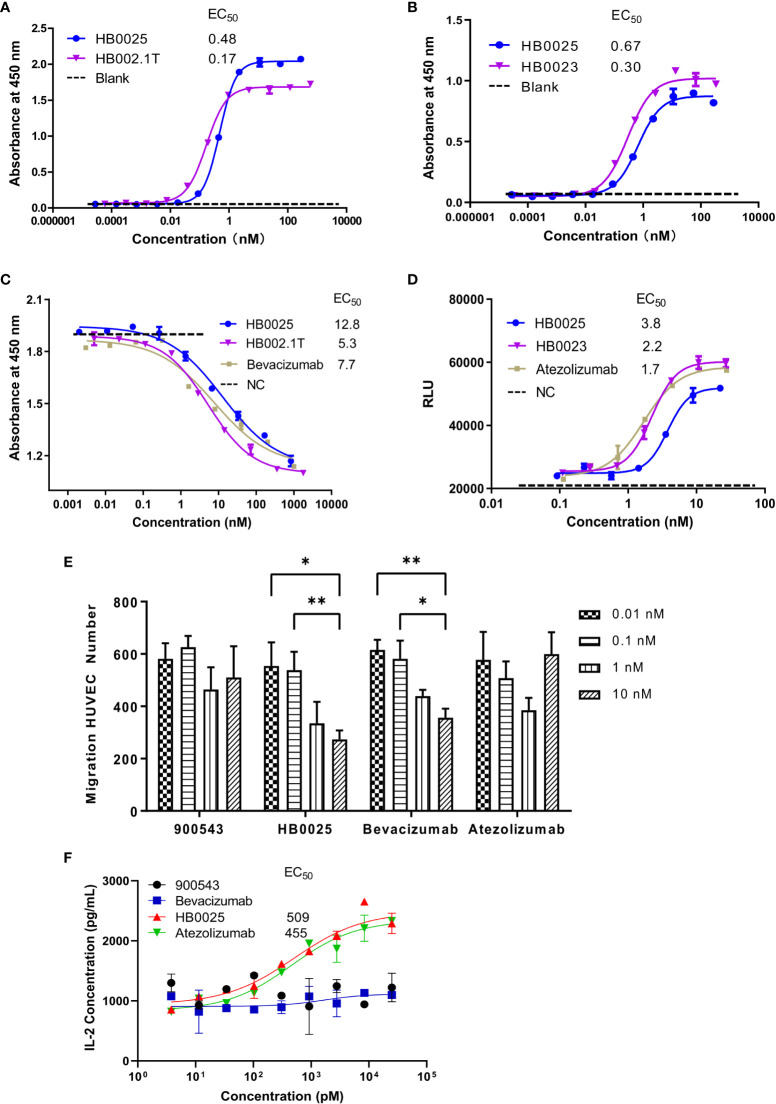
Figure 2.
The profile of binding affinity and blocking activity of HB0025, the parent molecules, and the market antibodies. (A) The ELISA binding activity of HB0025 and HB002.1T to VEGF. (B) The ELISA binding activity of HB0025 and HB0023 to PD-L1. The PBST was used as a blank sample. (C) The blockade effect of HB0025, HB002.1T, and bevacizumab on VEGF-induced HUVEC proliferation by the CCK-8 assay. Negative control (NC): The cells were treated with the mixture of VEGF solution and detection medium in the ratio of 1:1. (D) Inhibitory effect of HB0025, HB0023, and atezolizumab on the PD-L1 pathway by the luciferase reporter gene system. Negative control (NC): The cells were treated with a second antibody alone. (E) Inhibition of HUVEC migration by 900543 (negative control), HB0025, atezolizumab and bevacizumab (positive control); 0.01 nM and 0.1 nM were compared with 10 nM, *p < 0.05, **p < 0.01. (F) The dose-effect fitting curve of antibodies on the recovery of human IL-2 secretion, taking 900543 as the negative control. The data are representative of mean ± SEM; Experiments of (A–D, F) were performed in duplicate; data of Experiment E were derived from 5 visual fields.