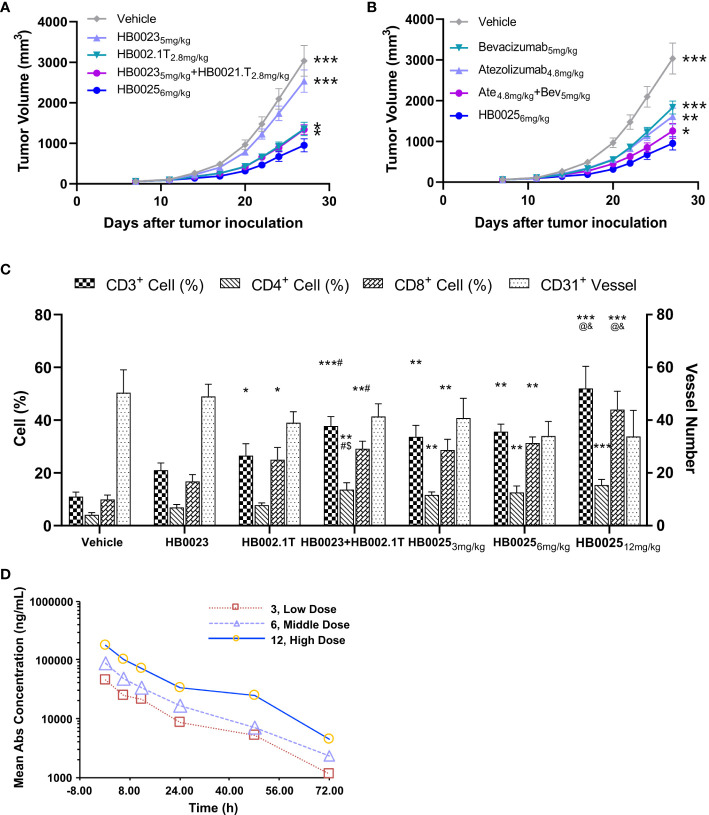
Figure 3.
Pharmacodynamics and pharmacokinetic analysis in NCG mice. (A) HB0025 treatment compared with HB0023, HB002.1T, and HB0023+HB002.1T in NCG mice. (B) HB0025 treatment compared with bevacizumab, atezolizumab, and bevacizumab+atezolizumab in NCG mice. *p < 0.05, **p < 0.01, ***p < 0.001 compared with HB00256mg/kg. (C) Tumor-infiltrating lymphocytes (CD3+, CD4+, CD8+) and the numbers of blood vessels (CD31+). *p < 0.05, **p < 0.01, ***p < 0.001 compared with the vehicle; #p < 0.05 compared HB0023 with HB0023+HB002.1T; $p < 0.05 compared HB002.1T with HB0023+HB002.1T; @p < 0.05 compared with HB0025 (3 mg/kg)/HB0025 (6 mg/kg) with HB0025 (12 mg/kg); and p < 0.05 compared HB002.1T+HB0023 with HB0025 (12 mg/kg). (D) The pharmacokinetic profiles of HB0025 in NCG mice at doses 3, 6, and 12 mg/kg. data expressed as mean ± SEM.