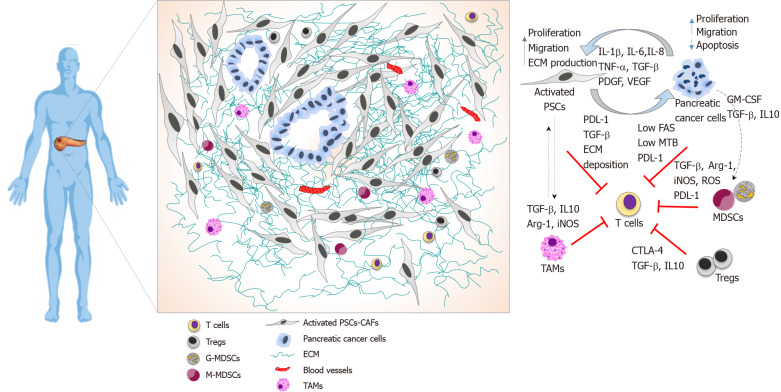
Figure 1.
Pancreatic ductal adenocarcinoma immune landscape and T cell immunosuppression. Illustrative image showing spatial localisation of T cells in the pancreatic ductal adenocarcinoma tumour microenvironment and cellular interactions that collectively prevent T cell infiltration and function. T cells are localised at the periphery of tumours preventing direct contact with cancer cells. Pancreatic stellate cells produce elevated amounts of extracellular matrix driving a fibrotic tissue that entraps infiltrated T cells, alongside with immunosuppressive cytokine to and expression of programmed death-ligand 1 (PDL-1). Pancreatic cancer cells avoid T cell killing by downregulating Fas, exhibiting low tumour mutational burden, expressing PDL-1 and secreting growth factors and cytokines that recruits immunosuppressive cells. Myeloid-derived-suppressor cells express PDL-1 and suppress T cells functions by several mechanisms, including depleting of arginase 1, the release of reactive oxygen species, and secretion of cytokines. Tregs directly suppress T cells, express cytotoxic T-lymphocyte-associated protein 4 and secrete cytokines. TAMs play a role in sequestering T cells at the periphery and secrete immunosuppressive cytokines. PSC: Pancreatic stellate cells; TAMs: Tumour-associated macrophages; CTLA-4: Cytotoxic T-lymphocyte-associated protein 4; GM-CSF: Granulocyte-macrophage colony-stimulating factor; Arg-1: Arginase 1; PDL-1: Programmed death-ligand 1; iNOS: Inducible nitric oxide; MDSC: Myeloid-derived-suppressor cells; ROS: Reactive oxygen species; ECM: Extracellular matrix; TBM: Tumour mutational burden.