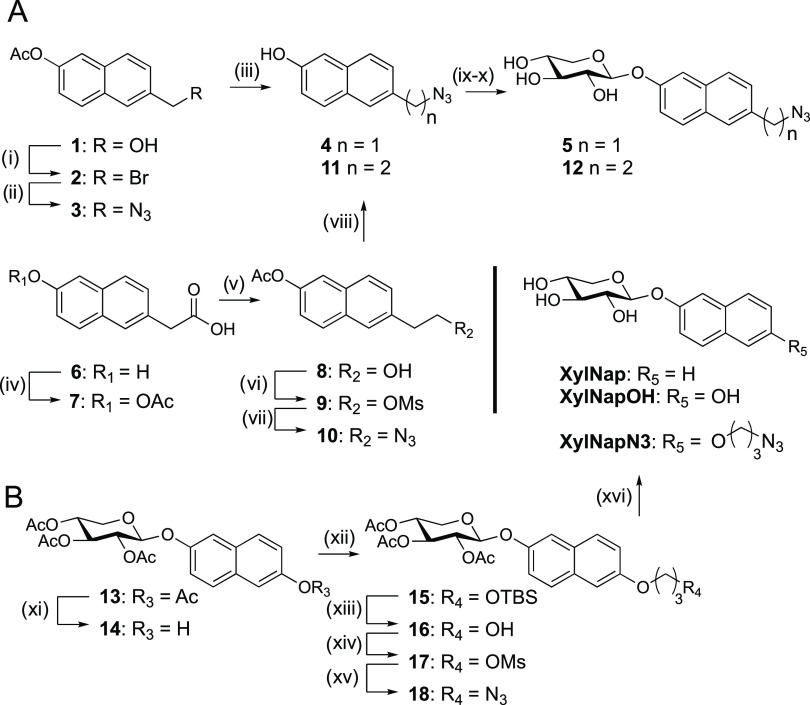
Scheme 1. Synthesis of Target Xylosides.
Reagents and conditions: (i) PBr3, CH2Cl2, 40 °C, 1.5 h, 56%; (ii) NaN3, DMSO, 40 °C, 2 h, 89%; (iii) 1 M NaOMe, MeOH, r.t., 2 h, 73%; (iv) acetic anhydride, pyridine, r.t., 24 h, 68%; (v) 2 M BH3·THF, THF, 0 °C to r.t., 20 h, 68%; (vi) MsCl, pyridine, 0 °C, 3 h, 79%; (vii) NaN3, DMF, 0 °C to r.t., 30 h, 74%; (viii) 1 M NaOMe, MeOH, r.t., 2 h, 89%; (ix) peracetylated xylose, BF3·OEt2, Et3N, CH2Cl2, 0 °C to r.t., then (x) 1 M NaOMe, MeOH, r.t., 1 h, 5: 13% over two steps; 12: 16% over two steps; (xi) NH4OAc, THF, MeOH, H2O, o.n., 40 °C, 84%; (xii) 3-(tert-butyldimethylsilyloxy)propyl bromide. K2CO3, DMF, Ar(g), o.n., 40 °C; then (xiii) HCl, MeOH, 30 min, r.t., 63% over two steps; (xiv) MsCl, pyridine, 1,5 h, 0 °C to r.t., 89%; (xv) NaN3, DMF, 30 min, MW heating at 90 °C, 88%; and (xvi) K2CO3, MeOH, 1.5 h, r.t., 84%.