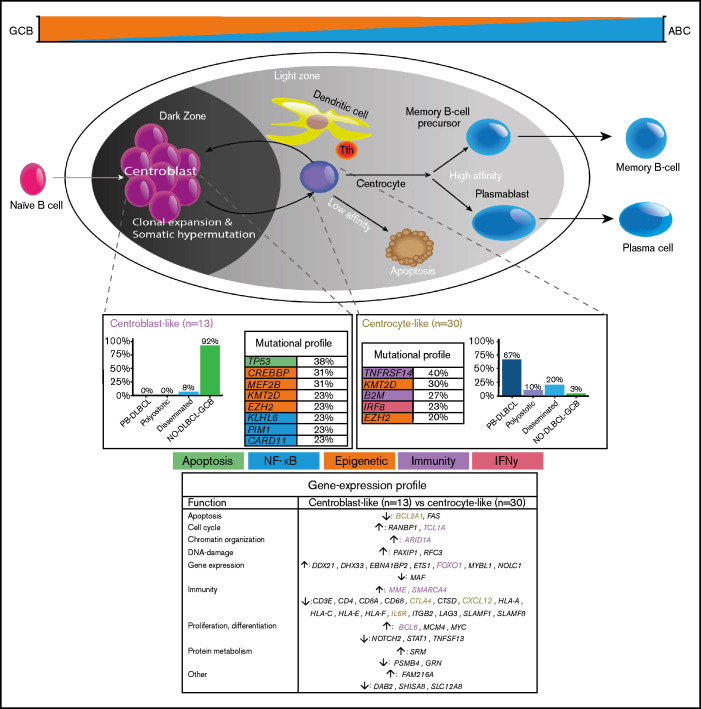
Figure 6.
Mechanistical overview of GCB development related to PB-DLBCL and NO-DLBCL-GCB and their identified specific GEP patterns and molecular profiles. As described by Li et al.,8 and corresponding with our GEP analysis showing an increased expression of BCL2A1 and IL6R, PB-DLBCL preferentially originated in the GC early LZ of B-cell development, indicating a centrocyte-like phenotype. The predominant centrocyte-like GCB phenotype in PB-DLBCL was subsequently supported by frequently mutated GCB-associated genes, such as B2M, EZH2, IRF8, and TNFRSF14, culminating in superior survival. In addition, PB-DLBCL exhibited significantly (P < .001) increased expression of immune response genes (CTLA4 and CXCL12), and together with frequent mutations in B2M and TNFRSF14, they are important for immune surveillance, suggesting that evasion from immune surveillance is crucial for PB-DLBCL to survive in their osseous environment. In contrast, upregulation of BCL6, MME, MYBL1, SMARCA4, and TCL1A suggested a centroblast-like constitution for NO-DLBCL-GCB. Accordingly, high expression of FOXO1, a centroblast hallmark and imperative for sustaining the GC DZ,62-65,69 was specifically identified in NO-DLBCL-GCB. Furthermore, elevated expression levels of ARID1A and SMARCA4 (both involved in chromosome organization) were found in NO-DLBCL-GCB. Together with frequent mutations in genes involved in epigenetics (CREBBP and MEF2B) and TP53 mutations, this indicates that, unlike immune evasion in PB-DLBCL, survival of NO-DLBCL-GCB is critically dependent on deregulation of chromosome organization and reduction of p53 activity. Our results thus emphasize that PB-DLBCL can be recognized as a distinct extranodal DLBCL, with a GCB-centrocyte-like phenotype, a specific GEP pattern, and a unique GCB-associated molecular constitution, reflecting favorable prognosis. Purple color indicates genes related to a centroblast-like phenotype, whereas brown-colored genes are related to a centrocyte-like phenotype.