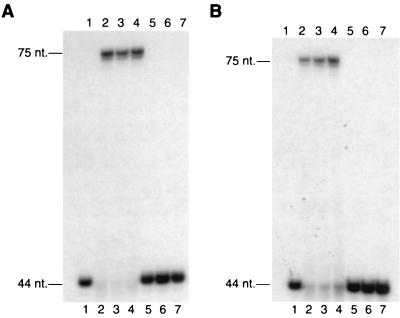
FIG. 2.
DNA polymerase and cis-syn T-T dimer bypass activities of C-terminally truncated Rad30 proteins. (A) DNA polymerase activities of the wild-type and truncated Rad30 mutant proteins were assayed by incubating the proteins (5 nM) for 10 min at 25°C with all four dNTPs (100 μM each) and the nondamaged DNA substrate (10 nM) as described in Materials and Methods, and reactions were stopped and analyzed on a 10% polyacrylamide sequencing gel. Lanes: 1, no protein: 2, wild-type Rad30 protein; 3, Rad30(1-578) protein; 4, Rad30(1-513) protein; 5, Rad30(1-452) protein; 6, Rad30(1-398) protein; 7, Rad30(1-340) protein. (B) T-T dimer bypass activities were assayed as described for the DNA polymerase activities in panel A, except that the DNA substrate used contained a cis-syn T-T dimer in the template strand at nucleotide positions 45 and 46 from the 3′ end.