Figure 3:
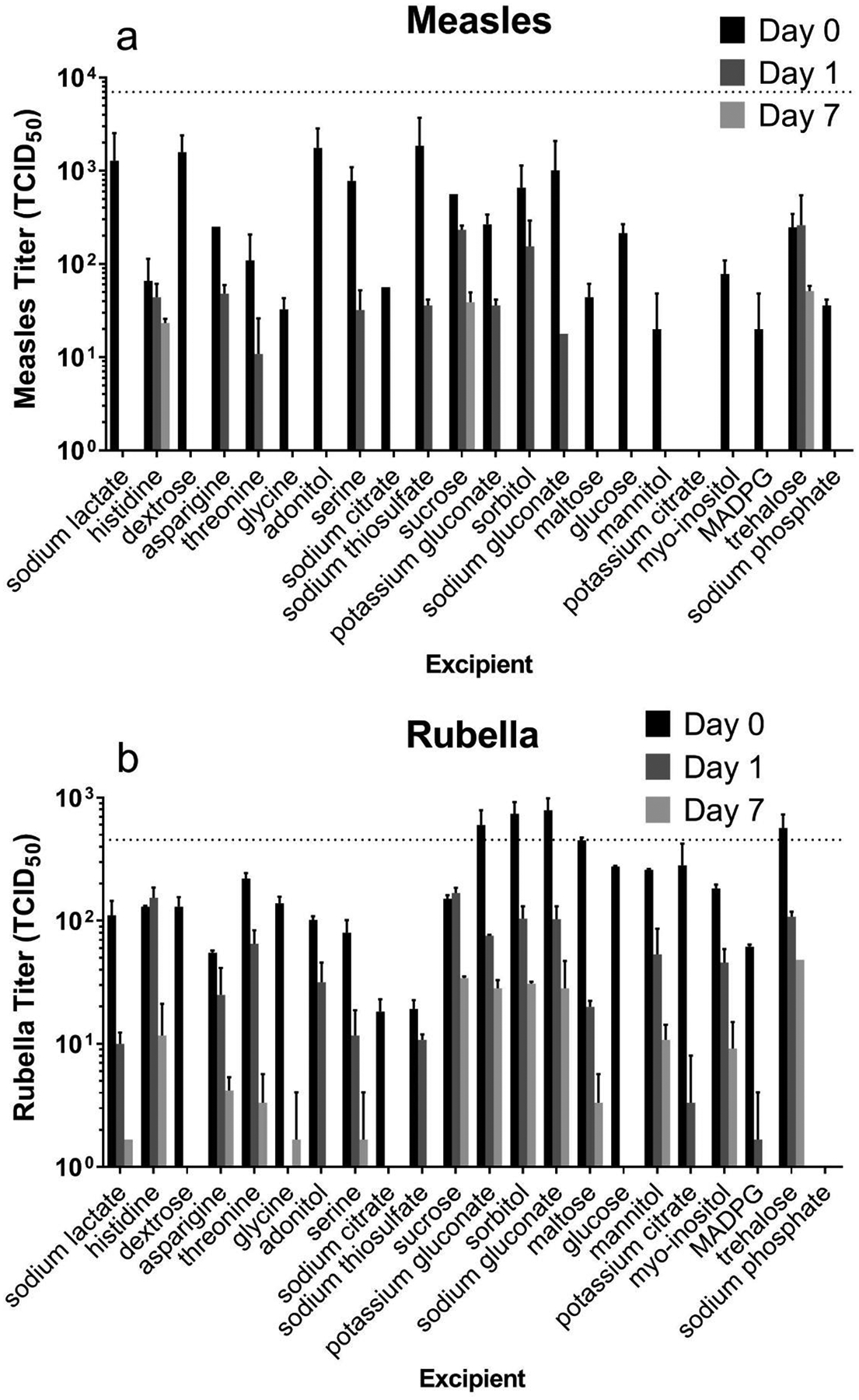
Effect of individual excipients on measles and rubella vaccine titer after drying and storage at 40°C for up to one week. Individual excipients were mixed with monovalent measles (a) or rubella (b) vaccine samples, dried as films for ~18 h at room temperature (20 – 25°C) and then stored at 40°C. All formulations contained 0.8 wt% potassium phosphate buffer (pH 7.5) and 1 wt% CMC. Day 0 represents stability after drying only. Titers were determined from 50 μl liquid or dried samples. The dotted lines indicate vaccine titer of liquid solutions before drying. Data are expressed as mean ± standard deviation based on 2 replicates each. No statistical analysis was performed on these data.
