Figure 5:
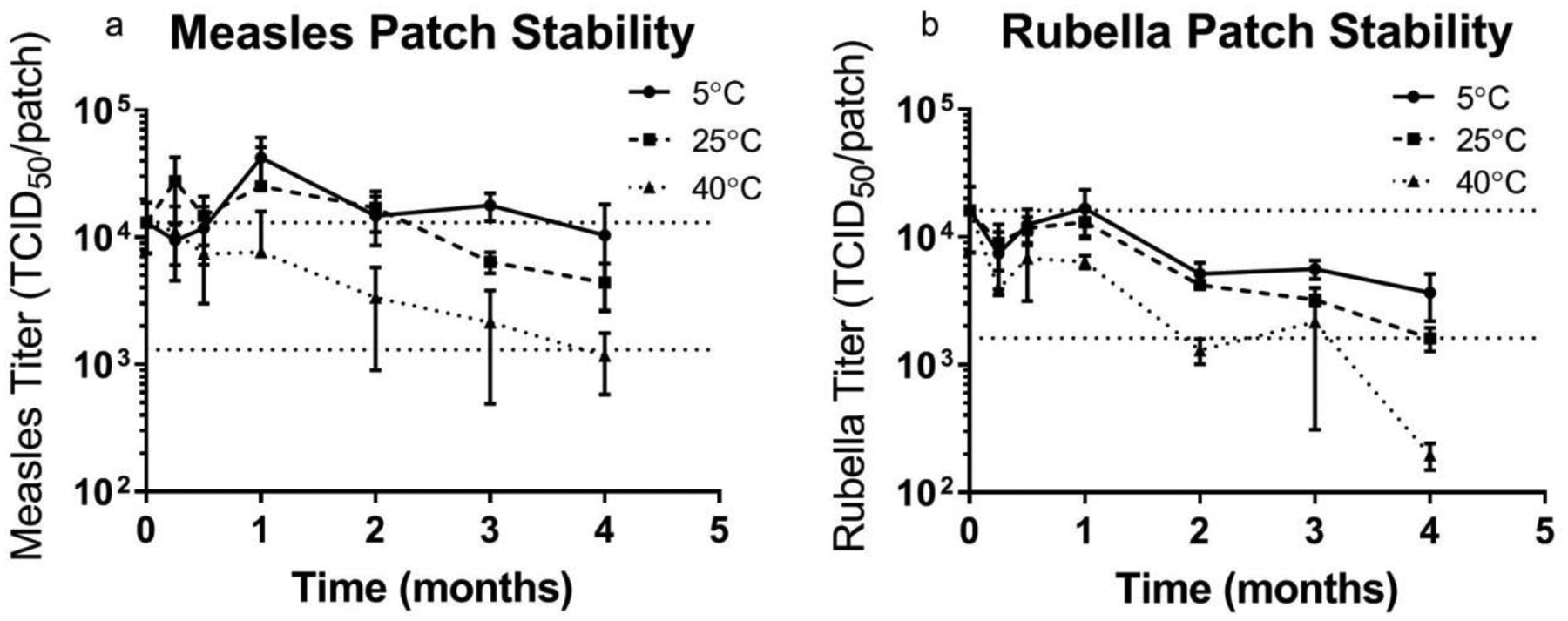
Stability of bivalent microneedle (MN) patches containing measles and rubella vaccines during extended storage. Patches were prepared using a formulation containing sucrose, threonine, and potassium phosphate buffer at pH 7.5 and then stored with desiccant at 5°C, 25°C or 40°C for up to 4 months. The upper horizontal dashed lines indicate vaccine titer of MN patches before storage and the lower horizontal dashed lines indicate vaccine titer equal to 10% of MN patch titer before storage. Data are expressed as mean ± standard deviation based on 4 replicates each. Statistical analysis was performed by Student’s t-test, with p < 0.05 considered significant; specific findings from this analysis are reported in the text.
