Abstract
Swimming in schools has long been hypothesized to allow fish to save energy. Fish must exploit the energy from the wakes of their neighbors for maximum energy savings, a feat that requires them to both synchronize their tail movements and stay in certain positions relative to their neighbors. To maintain position in a school, we know that fish use multiple sensory systems, mainly their visual and flow sensing lateral line system. However, how fish synchronize their swimming movements in a school is still not well understood. Here, we test the hypothesis that this synchronization may depend on functional differences in the two branches of the lateral line sensory system that detects water movements close to the fish’s body. The anterior branch, located on the head, encounters largely undisturbed free-stream flow, while the posterior branch, located on the trunk and tail, encounters flow that has been affected strongly by the tail movement. Thus, we hypothesize that the anterior branch may be more important for regulating position within the school, while the posterior branch may be more important for synchronizing tail movements. Our study examines functional differences in the anterior and posterior lateral line in the structure and tail synchronization of fish schools. We used a widely available aquarium fish that schools, the giant danio, Devario equipinnatus. Fish swam in a large circular tank where stereoscopic videos recordings were used to reconstruct the 3D position of each individual within the school and to track tail kinematics to quantify synchronization. For one fish in each school, we ablated using cobalt chloride either the anterior region only, the posterior region only, or the entire lateral line system. We observed that ablating any region of the lateral line system causes fish to swim in a “box” or parallel swimming formation, which was different from the diamond formation observed in normal fish. Ablating only the anterior region did not substantially reduce tail beat synchronization but ablating only the posterior region caused fish to stop synchronizing their tail beats, largely because the tail beat frequency increased dramatically. Thus, the anterior and posterior lateral line system appears to have different behavioral functions in fish. Most importantly, we showed that the posterior lateral line system played a major role in determining tail beat synchrony in schooling fish. Without synchronization, swimming efficiency decreases, which can have an impact on the fitness of the individual fish and group.
Summary statement
Giant danios loses their tail beat synchronization after their posterior lateral line system is ablated.
Introduction
Many fishes tend to form collective swimming groups, a behavior that is driven by factors including ecological niches, such as rivers, lakes and marine environments, and motivational states, such as feeding and mating. Fish schools are basic archetypes of the collective swimming groups, though they have various levels of organization, cohesive social structures, and neighbor-to-neighbor interactions (Chicoli et al. 2014; Marras et al. 2015; Ashraf et al. 2016). Forming a school benefits fish in many ways, which include predator avoidance and detection, increased foraging and spawning opportunities, and reduced energetic cost during locomotion. The latter has been the focus of recent research such that the benefit of forming optimized spatial structures in fish schools has been associated with large changes in locomotion and hydrodynamic efficiency (Partridge and Pitcher 1980; Inada and Kawachi 2002; Chicoli et al. 2014; Marras et al. 2015; Ashraf et al. 2016; Daghooghi and Borazjani 2016).
Schooling structures can be complex, and the optimization of fish schools for efficient swimming requires matching position, speed, and tail beat kinematics. For example, fish within schools can form diamond-shaped patterns (Weihs 1973), rectangular patterns (Daghooghi and Borazjani 2016; Novati et al. 2017; Verma et al. 2018), or phalanx patterns (Ashraf et al. 2017), but every structure requires some form of synchronization. In such school formations, many fish species are able to synchronize tail movements and reduce their tail beat frequency compared with swimming at the same speed on their own (Marras et al. 2015), an indication of a reduced energetic cost of locomotion. For example, a study on schools of Pacific mackerel (Scomber japonicus) showed fish had decreased tail beat frequencies and a decreased swimming effort, an indication of reduced energetic cost that often requires synchronization of tail beats (Takagi et al. 2013). Fish in trailing positions within schools of sea bass (Dicentrarchus labrax) showed lower tail beat frequency and a reduction in oxygen consumption rate (Herskin and Steffensen 1998) by positioning themselves in the wake of their neighbors and taking advantage of the vortices shed off of the tail. Studies on golden grey mullet (Liza aurata) also showed a decrease in tail beat frequency and less variation in tail beat amplitude regardless of spatial positions (Killen et al. 2012). Lastly, red nose tetras (Hemigrammus bleheri) completely synchronize their tail beats during high-speed swimming (Ashraf et al. 2016, 2017). As such, synchronization between neighbors is one of the crucial mechanisms involved to decrease energetic cost (Ashraf et al. 2016; Novati et al. 2017).
Most fish have a mechanosensory lateral line system that is important for schooling (Partridge and Pitcher 1980; Chicoli et al. 2014; Mekdara et al. 2018). The lateral line system allows fish to detect movement of the water closely around them. The basic sensory unit of the lateral line is the neuromast, which is composed of clumps of hair cells with stereocilia and kinocilia housed in a gelatinous cupula (Denton and Gray 1988; Kalmijn 1988; Coombs and Van Netten 2005; Coombs et al. 2014). These neuromasts are positioned either directly on the skin and are referred to as superficial neuromasts, or in bony canals or specialized scales along the body and referred to as canal neuromasts. Superficial neuromasts respond to water velocity, while canal neuromasts respond to pressure gradients or water acceleration (Kroese and Schellart 1987; van Netten and Kroese 1987; Denton and Gray 1988; Kalmijn 1988; Coombs et al. 2014).
Along with the different types of neuromasts, the lateral line system in most fishes is separated into two major regions: the anterior lateral line and the posterior lateral line (Coombs et al. 2014). Researchers have long hypothesized that these two regions may be specialized for different functions, based on morphological and fluid dynamic evidence. Typically, the anterior lateral line nerve separates into peripheral, dorsal, and ventral branches that innervate neuromasts along with the orbital, mandible, and up to the posterior regions of the head (Coombs et al. 2014). The posterior lateral line nerve is usually divided into two branches that innervate the main lines of neuromasts along the trunk. The basic arrangement of the lateral line system in most adult fish extends over much of the body, often with a larger proportion of neuromasts concentrated on the head (Chicoli et al. 2014; Webb et al. 2014; Ristroph et al. 2015; Carrillo and McHenry 2016), especially within the cranial canals.
A recent study on lateral line system distribution in rainbow trout (Onchorhynchus mykiss) found that there are more canal neuromasts on locations of the body that experience the strongest pattern of spatial and temporal pressure, which happens to be the anterior region (Ristroph et al. 2015). Ristroph et al. (2015) argue that this high concentration of neuromasts makes the head region more sensitive to variation in flow pressure, a pattern that is particularly important for swimming because the anterior lateral line is less affected by self-generated flow produced from the tail’s movement during swimming. In toadfish (Opsanus tau), the anterior lateral line could also enhance sound localization (Cardinal et al. 2018). Collectively, the arrangement of all neuromasts in the lateral line system seems to reflect critical fish behaviors such as feeding, rheotaxis, and predator detection, whereby fish require larger and more neuromasts in the head region for higher sensitivity to find food, evade predators, or navigate through a more complex, dense, or dark natural environment (Schwalbe et al. 2012; Ristroph et al. 2015; Carrillo and McHenry 2016). These studies provide evidence for functional differences between the anterior and posterior lateral line system within a species.
Most researchers study the role of the lateral line in fish by ablating the entire system with either aminoglycoside antibiotics or heavy metal ions. Surgically severing the lateral line nerve has been effective but is more invasive. Aminoglycoside antibiotics, such as gentamycin, and heavy metal ions, such as cobalt chloride, are ototoxic and are commonly used to ablate hair cells in neuromasts (Song et al. 1995; Harris et al. 2003; Van Trump et al. 2010; Butler et al. 2016). Once the hair cells are ablated and fish are removed from the chemicals, their hair cells regenerate over a few days to a week (Santos et al. 2006; Faucher et al. 2010; Pinto-Teixeira et al. 2015; Schwalbe et al. 2016; Mekdara et al. 2018).
Ablating the lateral line system does not prevent fish from schooling, but it does cause fish to school differently and in species-specific ways (Partridge and Pitcher 1980; Mekdara et al. 2018). In fish species (including Hemigrammus rhodostomus, Hemigrammus bleheri, and Devario aequipinnatus), ablating the entire lateral line system causes fish to swim at the same level and further away from their nearest neighbors (Faucher et al. 2010; Mekdara et al. 2018; McKee et al. 2020). Fish can also lose track of their neighbors and swim away from the school when their entire lateral system is ablated (Faucher et al. 2010; Mekdara et al. 2018) because it might be difficult to determine the heading of the fish beside it when the anterior lateral line system is ablated. Perhaps without the anterior lateral system as a forward antenna, fish are less able to respond to small changes from their nearest neighbor (Partridge and Pitcher 1980; Mekdara et al. 2018). In saithe (Pollachius virens), ablating the posterior lateral line system causes them to swim closer, more parallel, and at a higher elevation to their nearest neighbor (Partridge and Pitcher 1980). These studies show that the lateral line system plays a large role in determining the distance, heading, and position of an individual fish within the school.
In this study, we investigate the functional differences between the anterior and posterior lateral line in the organization and synchronization of giant danio, Devario aequipinnatus, in schools. We hypothesize that the anterior branch is mainly used for regulating position within the school, while the posterior branch helps maintain the synchronization of tail movements. Synchronized movements in fish schools rely on the sensitivity of each fish to the hydrodynamic pressure of the water and their surrounding neighbors. Previous studies have shown that the lateral line system plays an important role in determining school structures and cohesiveness of the school (Chicoli et al. 2014; Ashraf et al. 2016, 2017; Mekdara et al. 2018). Here, we show differences in synchronization when major regions of the lateral line were ablated. Ablating the anterior lateral line system causes changes in position and speed but ablating the posterior lateral line system completely removes tail beat synchrony. The anterior lateral line system might therefore function as a hydrodynamic antenna sensitive to position cues to keep track of neighbors. However, the posterior lateral line system might be more sensitive to changes in tail beat between the fish and its neighbors.
Materials and methods
Animals
Giant danios, Devario aequipinnatus (McClelland 1839; TL = 7.24 ± 1.12 cm, n = 136) were supplied commercially (LiveAquaria, Rhinelander, WI) and housed in groups of ≤25 fish per 40 L aquarium tanks (4 tanks) at 23°C, pH of 7.4, and a conductivity level of 385 µS. Fish were fed with goldfish flakes daily (TetraFin, Blackburg, VA) and kept on a 12-h:12-h light: dark cycle. All experiments followed approved Tufts University IACUC protocols M2012-145, M2015-149, and M2018-103.
Ablation of the lateral line system
The lateral line systems of giant danio were ablated using cobalt chloride, a heavy metal that is toxic to hair cells (Schwalbe and Webb 2014; Butler et al. 2016), or gentamycin, an aminoglycoside that ablates hair cells and inactivates transduction channels (Van Trump et al. 2010; Mekdara et al. 2018). Cobalt chloride and gentamycin leaves the hair cells of the inner ears intact and targets mostly the lateral line cells (Butler et al. 2016). To completely ablate the lateral line, we used either 0.001% gentamycin (for 24 h, n = 30; Sigma–Aldrich) or 1 mM cobalt chloride (for 4 h, n = 18; Santa Cruz Biotechnology, Inc., Dallas, TX) in 10 L aerated tanks that contained conditioned tank water and 0.01% tricaine methanesulfonate (MS-222, pH 7.4, Sigma–Aldrich). We changed to the cobalt treatment because it ablated the hair cells faster than gentamycin, which was important for the partial ablations described below. We found no differences between gentamycin and cobalt chloride treatments and, therefore, pooled (n = 48) the treatment groups for statistical analysis.
To partially ablate either the anterior and posterior lateral line system, we anesthetized fish using 0.02% buffered MS-222 in conditioned tank water and embedded the other portion of the fish’s body in a block of 4% low melting point agarose, which was heated to 85°C and cooled to 25°C for embedding (Carrillo and McHenry 2016). For the posterior ablation (n = 18), the head was embedded in agarose and a respiratory tube was inserted into the mouth of the fish before embedding. For anterior ablation (n = 18), the trunk was embedded in agarose. Once the agarose hardened and set, fish were placed into tanks with 1 mM cobalt chloride for lateral line ablation. Thus, only the anterior or posterior portions of the lateral line system that were exposed to the cobalt chloride were ablated, leaving the agarose embedded portions of the lateral line system intact. After the 4-h treatment, fish recovered for 6 h as they naturally remove themselves from the agar embedding. To evaluate the stressfulness of the embedding procedure, we also set up a sham treatment (n = 18), where anterior or posterior portions of the body were embedded into agar, but the exposed portions of their lateral line systems were in a solution of tank water with 0.01% MS-222. Ablation success was confirmed with fluorescent staining of the lateral line system.
Visualizing the lateral line system
To determine the viability of the hair cells of the lateral line system, we exposed representatives from each treatment group each week to a vital fluorescent dye in conditioned tank water (Magrassi et al. 1987; Mekdara et al. 2018). Fish were stained with 63 mM of 4-4(-diethtylaminostyryl)-1-methylpyridinium iodide (4-di-2-asp; Sigma–Aldrich) solution for 5 min immediately after behavioral experiments, and neuromasts were visually checked to see if they were intact. For quantitative imaging, we anesthetized fish with buffered 0.02% MS-222 and kept fish under with a respirator to pump water through the gills for 30 min. We quickly imaged the fish under a fluorescence microscope (Leica M165-FC, Lecia Microsystems, Wetzlar, Germany) using a GFP fluorescence filter (wavelength = 425/60 nm). Some fish (n = 12) were euthanized for high quality images at each experimental time point. Images were captured using a Nikon D3000 DSLR (Nikon Corporation, Tokyo, Japan) with the following camera settings: 2 s shutter speed, 1600 ISO speed, and a fixed aperture based on the fish size and the microscope’s magnification power (gain and white balance were set to automatic for low-light conditions). Depending on the size of the fish, between 150 and 300 micrographic images were captured to produce the highest quality images of the neuromasts. Micrographic images were auto-stitched together and blended (Adobe Photoshop CS, Ottawa, Ontario, Canada) to create a full view of the fish.
Experimental setup
Groups of giant danios were filmed in a large circular tank (120 cm diameter, 85 cm height) filled with tank water to a 60 cm depth (Mekdara et al. 2018). Ten fish from each housing tank were randomly selected for chemical treatment and tagged with visible implant elastomers, which left a colored mark on the dorsal region 7 days before experiments (Northwest Marine Technologies, Tumwater, WA; Olsen and Vøllestad 2001). Experimental trials were recorded at three time points: 1 week before treatment to ablate all or part of the lateral line, immediately after treatment, and 2 weeks after treatment. Each treated fish was kept with four untreated fish (= control fish) during the experimental stage. Fish were transferred from their treatment tank with a net and carefully placed into the testing tank (pH 7.4, temperature at 23°C, 385 µS conductivity level). Fish spent 15 min acclimating to and exploring the tank before the initial recordings began. Fish swam in three 5-min trials. However, in order to record high resolution images of the behavior, only 20 s of the 5-min trials were captured because of limited data storage of the high-speed cameras.
Schooling experiments
Fish schooling behavior was filmed at 50 frames per second with two top-view high-speed cameras (Phantom M120, Vision Research, Wayne, NJ; PCO.edge, PCO AG, Kelheim, Germany) synchronized to a common trigger (Supplementary Movies S1–S4). The camera’s focal lengths, distortions, and field of view were calibrated using easyWand (Theriault et al. 2014). We reconstructed the 3D trajectories and tail motion of each fish in the school with DLTdv5 open-source software (Hedrick 2008) by tracking the snout and tail position of each fish. We used custom software in Matlab (MathWorks, Natick, MA) to extract the coordinate position for data analysis.
Analysis of schooling behavior
We used digitized videos to calculate the distances between each fish and all other fish, using the same metrics as in our previous study (Mekdara et al. 2018). Specifically, we calculated the bearing in the horizontal plane (θ) and elevation angles (ϕ), and nearest neighbor distance (NND) from the 3D coordinates determined by the computer tracked videos using similar calculations from Partridge (Partridge and Pitcher 1980). Schooling tendency, or time spent swimming in the main group of a fish in a trial, was determined by calculating the distance of the treated fish from the geometric center of the 3D volume of the school. We chose a threshold distance of 1.5 times the mean distance of an untreated fish from the center of the school. The mean distance was 0.72 ± 0.13 total body lengths (Fig. 4). We quantified the fraction of time that the fish spent swimming within the threshold distance as a measure of its tendency to school.
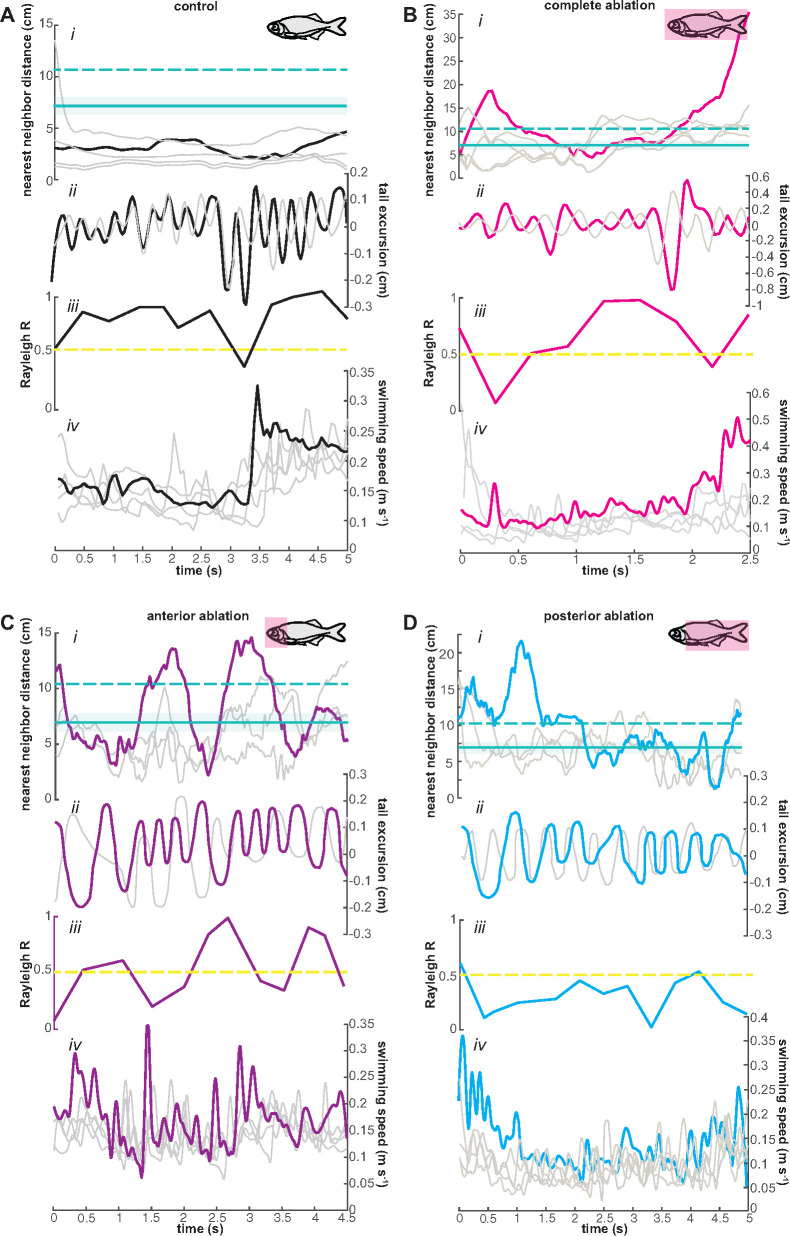
Fig. 4.
Differences in schooling behavior when major regions of the lateral line are ablated. Representative trials showing (i) nearest neighbor distance, (ii) tail excursion phases, (iii) tail beat by tail beat R coefficient with synchrony threshold of 0.5 (dashed yellow line), and (iv) swimming speed. (A) Pretreatment fish (control) usually stay within the school and have higher tail beat synchrony. Dashed green line represents the in-school threshold distance and the solid green line with the shaded error region represents the mean body length of giant danios (BL = 7.24 ± 1.12). The tail beat frequency increases as fish swim in and out of the school. (B) Treated fish with their lateral line system completely ablated usually stay within the school, but sometimes swim away from the school and have some trouble with tail beat synchronization. (C) Treated fish with anterior lateral line ablation swims in and out of the school and loses tail beat synchrony. Fish with posterior lateral line ablation have trouble synchronizing their tail beats.
For tail beat synchrony and overall tail beat analysis, we calculated the tail excursions by determining the heading of the fish on the horizontal plane (Fig. 1). The lateral excursion of the tail is the position of the tail normal to the heading (Fig. 1A). We then calculated the tail frequency from the lateral excursion by identifying the number of peaks throughout the entire tail beat cycle. If the peaks from the lateral excursion of the focal fish are in phase or directly out of phase with its nearest neighbor, the tail beats would be considered synchronized or phase-locked (Fig. 1B).
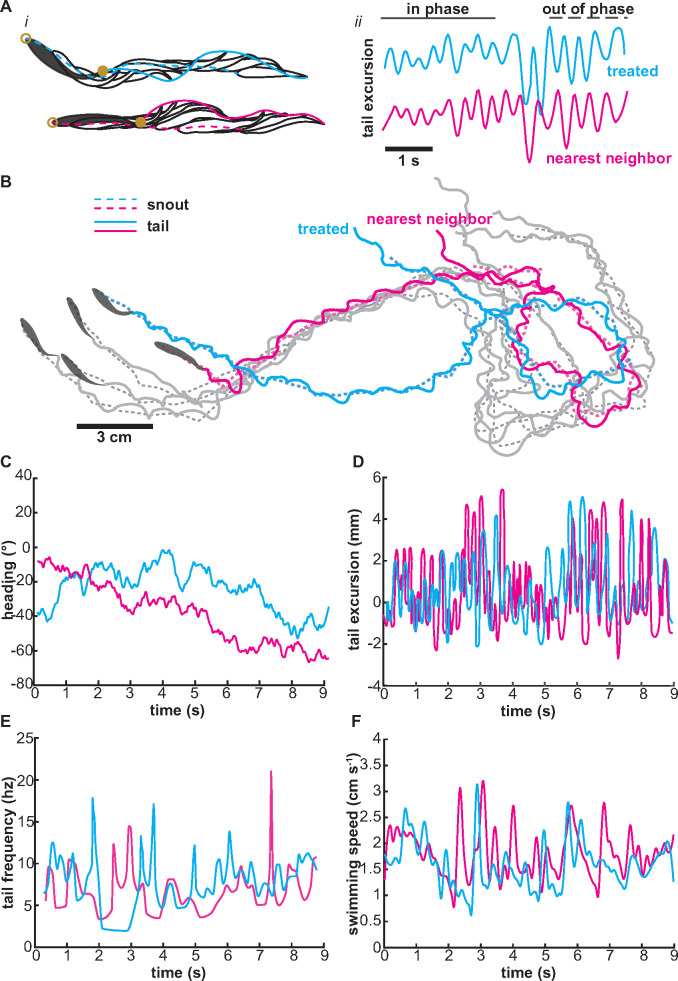
Fig. 1.
Quantification of tail synchrony in schooling giant danios. (A) Calculation of heading and tail excursion. (i) Trajectory of a fish and its nearest neighbor showing heading (open circle, dotted line) and tail trajectory (closed circle, solid line). (ii) Tail synchrony as determined by phase locking (in-phase or out of phase) of the tail’s lateral excursion between treated fish and its nearest neighbor. (B) Schooling trajectory of a group of fish with snout (dotted line) and tail (solid lines) tracks. Calculated (C) heading, (D) tail excursion, (E) tail frequency, and (F) swimming speed of the group of fish in panel B.
Statistical analysis
Our experiment had a repeated measure design. For each target fish, the nearest neighbor distance, schooling tendency, volume, and distance from the school were tested to find significant differences among the experimental weeks. All data were tested for normality and uniformity using JMP Pro 13 (SAS Institute Inc., Cary, NC). Further statistical analyses were done using the Statistical Toolbox in MATLAB (Mathworks). A generalized linear mixed model approach (GLMM) (MATLAB—MathWorks) was used to analyze the nearest neighbor distance, schooling tendency, volume, and distance from the school where the weeks were considered as a fixed factor variable and the trial number as a random factor. This approach allowed for the selection of random (trials) and fixed effects (pretreatment vs post-treatment) while addressing the time or repeated measures for each individual. Since we were mainly interested in the comparison between the pretreatment group and the treated and post-treated groups, we used Dunnett’s multiple comparison test (Dunnett 1955) to test the significant differences of the post-treatment trials, relative to the results from the pretreatment trials.
To quantify tail beat synchronization, we used custom MATLAB code (MathWorks) to identify the time of peaks in lateral excursion of the tail for each fish. The tail excursion was calculated by measuring the overall lateral excursion of the tail from the horizontal plane with respect to the fish’s heading. Tail beat synchrony was calculated by overlapping the nearest neighbor fish’s tail excursion with the treated fish’s tail excursion to determine tail beat by tail beat phase differences. We then calculated the phase angular mean, standard deviation, and mean vector length () for the phase differences between tail beats. We calculated for each trial and used a Rayleigh test for circular uniformity. Significance of the Rayleigh test shows both one-sidedness and concentration of the directions around the angular mean (Batschelet 1981). To test the differences between the two samples, we used a Watson–Williams F-test, which test for differences in the mean and angular variance (Batschelet 1981). We also used a χ2 test for binned angular data to compare the overall angular distribution between each treatment week.
All statistical tests were considered significant at (Table 1). Values are reported as means ± standard deviation, with sample size, and the value from the statistical test. Angular data are reported as angular means ± standard error of means, with sample size n, , and the value from the statistical test.
Table 1.
Results of statistical tests for differences across treatments
| Dependent variable | df | F | P-value |
|---|---|---|---|
| Schooling tendency (%) | 6 | 22.526a | <0.001 |
| Nearest neighbor distance (BL) | 6 | 14.158a | <0.001 |
| Bearing (deg) | 6 | 6.125b | 0.047 |
| Elevation (deg) | 6 | 1.43b | 0.057 |
| Speed (BL s−1) | 6 | 23.097a | <0.001 |
| Frequency (Hz) | 6 | 11.776a | <0.001 |
| Rayleigh R (coefficient) | 6 | 67.677a | <0.001 |
Treatments are pre-treatment, complete, anterior, or posterior ablation immediately after treatment, and complete, anterior, or posterior ablation 2 weeks after treatment. BL, body length; df, degrees of freedom.
Results of an overall statistical test using the linear mixed model approach.
Results of an overall statistical test using the Watson–Williams F-statistics for angular data.
Results
A stereoscopic camera setup was used to record and track the 3D positions of giant danios swimming around a large circular tank. Snout and tail position of each fish in the school were tracked to characterize the heading and their tail excursion relative to their heading (Fig. 1A). Figure 1 shows the metrics used to determine tail synchrony between a treated fish and its nearest neighbor fish. Supplementary Movies S1–S4 show the example of schooling behavior in the pretreatment and treatment weeks.
Anterior or posterior lateral line systems were isolated and ablated
We used a fluorescent dye (4-di-2-asp) to visualize functional neuromasts after ablation. Functional neuromasts were observed on the head and trunk of normal giant danios after fluorescent staining (Fig. 2A). On the head, both canal neuromast and superficial neuromasts were located around the eye orbit, along the mandible and in lines on the operculum. Large clusters of superficial neuromasts were located above the eye (Fig. 2Ai) and anterior to the naris. Canal neuromasts were located in bony canals lining the eye orbit, and the ventral edge of the mandible. Along the trunk, a long canal extends from the head near the dorsal edge of the operculum and extends ventrally along the body to the caudal fin. Both canal and superficial neuromasts were located on the trunk canal (Fig. 2Aii).
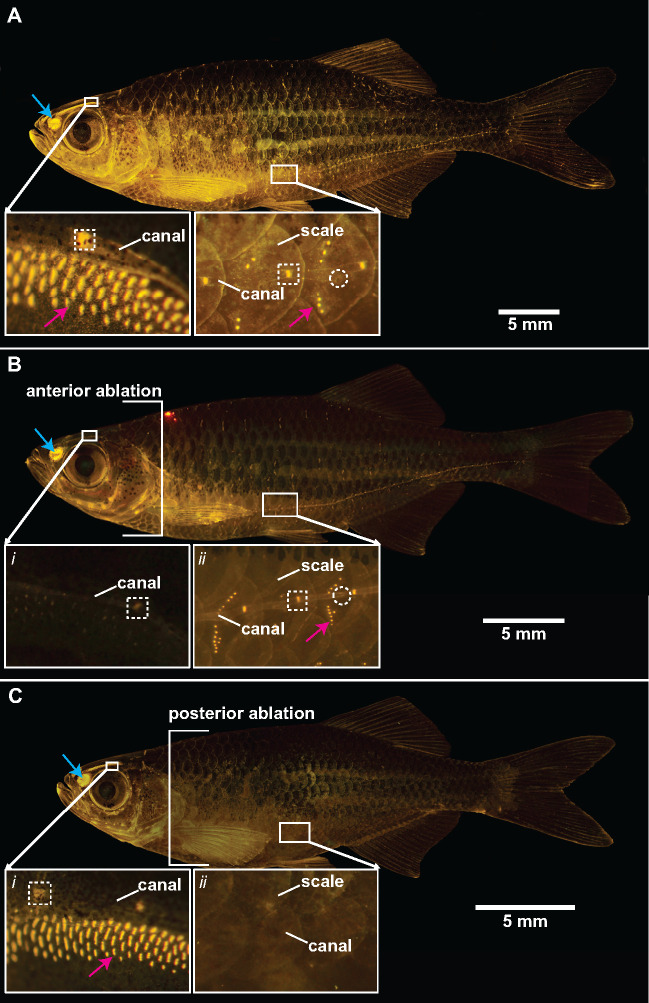
Fig. 2.
Fluorescent staining confirms lateral line ablation treatments in giant danios using cobalt chloride. Adult giant danio stained with 4-di-2-asp, showing metabolically active neuromasts of the lateral line system as bright yellow dots. (A) Before ablation, (B) anterior head ablated, and (C) posterior trunk ablated. In each panel, the inset figures show close-ups of the lateral line system in approximately the same regions on the head and trunk. Canal neuromasts shown in white dashed boxes, superficial neuromasts indicated with arrows (red), and white-dashed circles highlight canal pores. Also present are labeled olfactory cells (blue arrow). Scale bars, 5 mm.
The cobalt chloride treatment successfully ablated exposed hair cells in giant danio lateral line system but not in the areas protected by agarose (Fig. 2B and C). Lack of fluorescent staining in treated fish demonstrates anterior ablation (Fig. 2B) and posterior ablation (Fig. 2C). However, cobalt chloride did not disable olfactory hair cells, indicated by positive 4-2-di-asp staining. Neuromast staining returned to pre-treatment levels after 1 week and indicates that neuromasts were fully regenerated 1 week after ablation, as in our previous study (Mekdara et al. 2018).
Treated fish spend less time in the school and swim further from their neighbors
Immediately after the treatment, fish with completely ablated lateral lines were able to remain in a school with other fish. Fish with regional ablation in the anterior or posterior lateral line spent much less time in the school than control fish or those with completely ablated lateral lines (Fig. 3 and Table 1, P < 0.05). One week after treatment when the hair cells regenerated, all groups continued to spend much less time in the school (Fig. 3 and Table 1, P < 0.05).
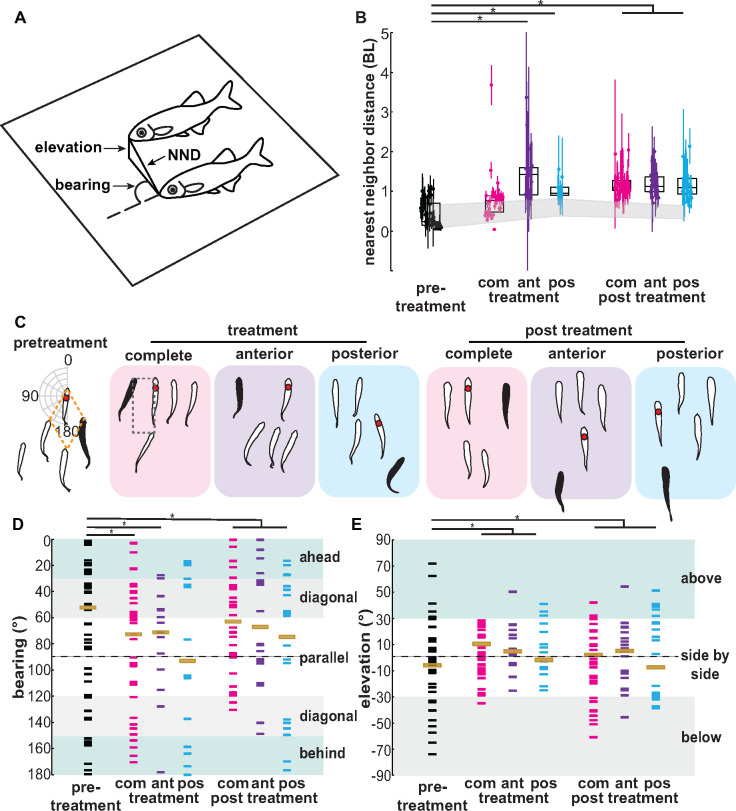
Fig. 3.
Ablation of the lateral line causes changes in relative position and distance, depending on region ablated. (A) Representation of two nearest neighbor fish in a school. Schematic diagram shows the nearest neighbor distance (NND), bearing (, and elevation. (B) Immediately after treatment, nearest neighbor distance (NND) increases for anterior (ant) and posterior (pos) lateral line ablated fish, but not for fish with their lateral line completely (com) ablated. In panel (B), box plots show the median and 25th and 75th percentiles, colored points represent the mean and standard deviation for an individual fish in each trial, and the gray-shaded region represents the sham-treated fish. (C, D) Bearing of the nearest neighbor fish (red dot in panel C) changes after ablation of the lateral line. The data range of the mean bearings of each nearest neighbor fish is plotted on a polar plot from 0° (swimming in front) to 180° (swimming behind) with respect to treated fish (black shaded in panel C). Outline of diamond (orange dashed-line) and box (black dashed-line) formation is shown. (D) Overall distribution of the mean bearings in each binned areas (ahead or behind, diagonal, and parallel). (E) Fish swam at similar elevations side by side in all conditions for most of the time, but their overall distribution is narrow in the side-by-side binned formation rather than above or below their nearest neighbor. For binned angular data, statistical significance was determined by tests that assess significant differences in overall distribution. Asterisks (*) indicates P < 0.05.
Control fish in the pretreatment week tended to swim very close to one another (Fig. 3B). Immediately after treatment and with a complete lateral line ablation, the mean nearest neighbor distance for the treated fish increased but was not significantly different from the control. Fish in this treatment group tended to swim with the school, but at a further distance. The treated fish that had either their anterior region or posterior region ablated increased their distance significantly compared with control (P < 0.05). This also led to them spending much less time in the school as they would lose track of their neighbors and end up swimming alone (Figs. 3B and 4C and D, P < 0.05). During lateral line regeneration, treated fish 2 week after the ablation treatment remained at further distances to their nearest neighbors for all treatment groups and were significantly different than control fish (P < 0.05). The sham treatment had no significant effect on the nearest neighbor distance at any point throughout the experiment (Fig. 3).
Immediately after ablating the lateral line system, fish swam at a faster mean speed (Fig. 5A, P < 0.05), which implies a higher tail beat frequency (Bainbridge 1958). During the regeneration period, the mean speed also increased significantly (P < 0.05). However, in each condition, the schooling group did not increase their speed. Sham treated fish swam at the same speed as control (Fig. 5A).
Fig. 5.
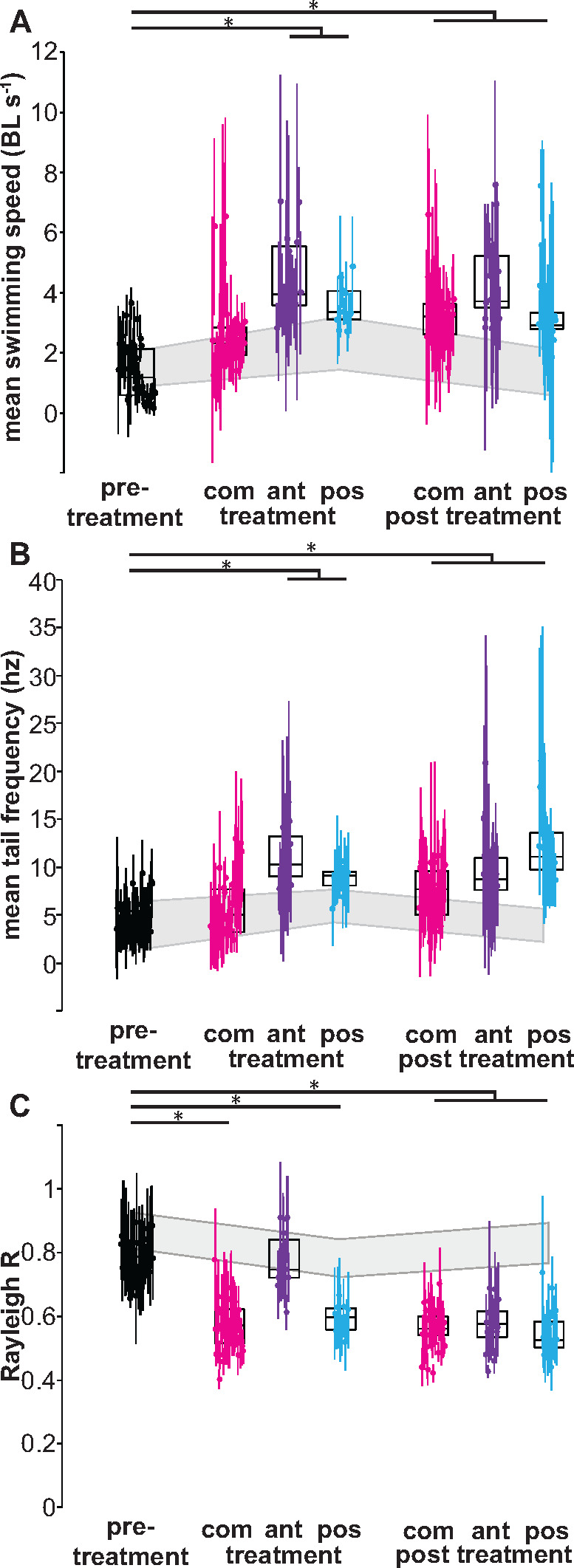
Tail beat synchrony decreases when the posterior lateral line system is ablated. (A) Swimming speed increases when the anterior or posterior lateral line is ablated. (B) Tail beat frequency increases when the lateral line is ablated, especially for fish with the anterior (ant) and posterior (pos) region ablated. (C) Rayleigh R calculation for a fish’s tail excursion relative to its nearest neighbor. R decreases when the lateral line system is ablated. The effects remain when the lateral line is regenerated. Asterisks (*) indicates P < 0.05.
Treated fish changed their angular position to their nearest neighbors
Ablating any portion of the lateral line system also affected the angular position of the treated fish in the school. We quantified the mean bearing, , of each nearest neighbor fish relative to the treated fish in the horizontal plane to determine the schooling structure. A bearing of 45° or 135° indicates the diamond formation, while a bearing of 0°, 90°, or 180° indicates the box formation (Fig. 3C–E). Ablating the anterior or complete lateral line resulted in fish positioning themselves more parallel to their neighbors (Fig. 3C and D, P < 0.05), but fish with a posterior ablation maintained more of a diamond pattern, similar to control fish. Without treatment, giant danios mostly adopted a diamond school formation, with each fish following diagonally ahead or behind and to the side of their nearest neighbor (Fig. 3C). One week after treatment, all treated fish tended to swim in a box formation, directly beside or directly behind their neighbors, though posterior ablated fish fluctuated between the box and diamond formation (Fig. 3C and D, P < 0.05). Sham-treated fish did not vary substantially from control fish.
To specifically examine changes in school formation from diamond to box patterns, we binned the range of bearings into three ranges with equal areas and compared the distribution (left and right side combined) between the control and all treatment weeks (Mekdara et al. 2018). The three ranges are the following: (1) fish that swam either directly ahead or behind their neighbors (θ = 15 ± 15° or 165 ± 15°), (2) fish that swam in a diamond formation (θ = 45 ± 15° or 135 ± 15°), and (3) fish that swam directly beside their neighbors (θ = 90 ± 30°) (Fig. 3C and D). We found significant differences between binned bearing angles (P < 0.05). Control fish spent the most time in a diagonal formation, but also swam at all bearing ranges (Fig. 3D). Giant danios that had their lateral lines ablated in either region increased the amount of time spent in a box formation when compared with the control group (P < 0.05). The anterior ablated fish and the fish with their entire lateral line ablated swam in similar positions closer to 90° angles (P < 0.05). Fish with their posterior lateral line ablated swam at 90° angles, but also adopted a staggered formation (Fig. 3D). These formations continued into the following week after regeneration of hair cells.
The mean elevation between lateral line ablated fish and their neighbors was not affected by the treatment (Fig. 3E), but the distribution of elevations did change significantly. Mean elevation between treated fish and control fish did not differ at any week (Fig. 3E), but the standard deviations decreased significantly with treated fish, with fish spending much more of their time in the same plane as their neighbors. Two weeks after treatment, treated fish also spent more time side-by-side when compared with control fish.
The posterior lateral line is necessary for tail beat synchronization
Control fish often synchronized their tail beats with their nearest neighbor (Fig. 5A but also see Fig. 1). This tail synchrony or phase-locking can be quantified by comparing the tail beat frequency, which must match during synchrony, and the Rayleigh R statistic across experiments (Fisher 1993). An R statistic based on the numbers of tail beats in each trial in our data set that was close to 1 indicated synchrony and an R-value below ∼0.5 indicated unsynchronized movements. Fish with a completely ablated lateral line lost synchrony, but often did not increase their tail beat frequency as much as the other treatments (Figs. 4B and 5C and D, P < 0.05). Fish with anteriorlateral line ablated had higher average tail beat frequency than most control fish, but when their frequency matched their neighbors, they managed to synchronize their tail beats with their neighbors (Fig. 5B and C). This fluctuation is due to their behavior of swimming in and out of the school (Fig. 5C). Immediately after ablating the posterior lateral line system, fish swam with a higher tail beat frequency and a lower synchronization with their neighbors (Figs. 4D and 5, P < 0.05). In general, all treated fish swam faster, as would be expected with a higher tail beat frequency. After regeneration of the lateral line system, tail beat frequency for all treatment groups remained significantly different and were less synchronized compared with control fish, while those with complete ablations were closer in both frequency and synchronization to control fish (Fig. 5, P < 0.05). In general, fish that swam at a further distance from their neighbors, either away or catching up with the school once they lose sight of it, tended to be less synchronized with the neighbors (Figs. 4 and 5, P < 0.05).
Discussion
For fish to school, they must form groups and synchronize their movements with their neighbors (Ashraf et al. 2016), behaviors that both rely on sensory systems and especially the flow sensing lateral line system. Schooling helps save energy, and without synchronization of movements within the school, this energy savings can decrease (Ashraf et al. 2016, 2017). In our previous study, we showed that giant danios with their lateral line system ablated can still school, but their overall position within the school changes. Fish swam further from their neighbors and more in a side-by-side pattern (Mekdara et al. 2018). In this study, we examined the functional differences between the two branches of the lateral line system: the anterior lateral line, which consist of neuromasts on the head, and the posterior lateral line, which consist of neuromasts on the trunk and tail. We have shown using partial ablations of the lateral line system using cobalt chloride that schools of giant danio, Devario aequipinnatus, require the posterior lateral line to synchronize their tail beats during free swimming. When their posterior lateral line was ablated, treated fish lost synchrony with their neighbors (Figs. 4D and 5). In contrast, when the anterior lateral line was ablated, treated fish could maintain synchronization but tended to swim further from their neighbors or lose the school entirely (Figs. 4C and 5). When either portion was ablated, fish had trouble staying within the school (Fig. 4B).
We also examined the behavioral changes as the lateral line system regenerates. After chemical ablation, hair cells regrow and recover function in less than 1 week (Harris et al. 2003; Pinto-Teixeira et al. 2015; Schwalbe et al. 2016; Mekdara et al. 2018; Cruz et al. 2015). However, in our previous study, we found that behavioral changes persist for up to 8 weeks after ablation (Mekdara et al. 2018). We hypothesized that the afferent nerve could be hypersensitized after the ablation, or perhaps that it could regrow in a different way (Mekdara et al. 2018). Because of these observations, in this study, we also considered the functional changes 2 weeks after ablation. At this time, when the hair cells have fully regrown, we find that fish with any portion of the lateral line ablated still have trouble staying in a school (Fig. 4 and Table 1) and maintain a larger distance to their neighbors (Fig. 3B and Table 1). Fish with only the anterior lateral line nerve ablated, however, were able to synchronize their tail beats with their neighbors, while, 2 weeks after treatment, those with the posterior lateral line ablated still could not synchronize (Fig. 5B and C).
Our results for partial ablations may seem contradictory to those from complete ablations. In particular, immediately after treatment, fish with a completely ablated lateral line maintained the same nearest neighbor distance as control fish, as expected (Partridge and Pitcher 1980; Faucher et al. 2010; Mekdara et al. 2018), but fish with partially ablated lateral lines swam farther from their neighbors (Fig. 3B). When only the anterior or posterior regions of the lateral line was ablated, giant danios swam at greater distances between their neighbors and changed their position within the school, spending more time directly beside and at the same elevation as their neighbors (Fig. 3). We suggest that the differences between the behavior of fish with partial ablations compared with complete lateral line ablation are due to the sensory conflicts between the intact and ablated portions of the lateral line in the partially ablated individuals. As in our previous study, vision can be used to maintain the schooling structure, even with a completely inactivated lateral line system (Mekdara et al. 2018). Both vision and the lateral line system can be used to regulate distances between neighbors or maintain a preferred nearest neighbor distance (Partridge and Pitcher 1980; Faucher et al. 2010; Middlemiss et al. 2017; Mekdara et al. 2018; McKee et al. 2020); hence ablation of the entire lateral line system does not cause fish to swim at a different distance to the neighbors, but it does cause them to swim at different angles and positions within the school. The differences in angles may be related to impairments in sensing overall swimming direction (Oteiza et al. 2017), but we suggest that the main effect is that fish move to have a more advantageous location for better visual cues to track neighboring fish (Pitcher et al. 1976; Partridge and Pitcher 1980). In contrast, when only a region of the lateral line system is ablated, the mismatch in sensory information from the intact and ablated portion may cause more difficulties than the complete lack of lateral line sensation. There may also be sensory conflicts between visual cues and the intact and ablated portions of the lateral line system.
Multisensory information from the lateral line and vision, as well as other senses, are processed in midbrain regions, including the optic tectum (called the superior colliculus in mammals) and the nucleus medial longitudinal fasciculatus (nMLF) (Coombs and Montgomery 2014; Coombs et al. 2020). These regions integrate multisensory information to help orient toward flow or maintain position in a school (Coombs et al. 2014). Multisensory integration has often been studied by completely disabling one sense (e.g., weakly electric fish tracking a refuge in the light and in the dark: Stamper), which means that relatively little is known about how fish process multisensory conflicts. However, Sutton et al. suggested that fish combine multisensory information linearly, but they dynamically alter the gain of the information based on the salience of the inputs. Similar processes were identified in hawkmoths that received conflicting mechanosensory and visual information (Roth et al. 2016). For our study, this relatively simple process of linearly combining sensory inputs with different weights may not be sufficient to compensate for partial or unreliable lateral line information.
Like previous studies, our results indicate that vision can be used to maintain the schooling structure, even with a completely inactivated lateral line system (Pitcher et al. 1976; Partridge and Pitcher 1980; Mekdara et al. 2018; McKee et al. 2020). Both vision and the lateral line system can be used to regulate distances between neighbors or maintain a preferred nearest neighbor distance (Partridge and Pitcher 1980; Faucher et al. 2010; Middlemiss et al. 2017). However, sensory conflicts might arise if information from the lateral line system is unreliable during regeneration, as the fish may need time to readjust (see Mekdara et al. 2018). Even with a newly regenerated lateral line system, fish still had trouble matching speed and swam more parallel and at faster speeds than control (Figs. 3B and 5A). Fish occasionally lost sight of their school and swam away from them, which was likely caused by conflicting information from the lateral line system. Our results showed that fish probably relied more on vision as their structures remained different from normal control positions and their nearest neighbor distances remained large (Figs. 3 and 4, but also see Fig. 5).
The differences between the treatment conditions demonstrate that the anterior and posterior lateral line system have different functions. Other studies have suggested that the anterior lateral line system is mainly used for local field detection such as feeding and prey movements (Nair et al. 2017; Carrillo et al. 2019) or to enhance auditory cues (Cardinal et al. 2018). With the anterior lateral line ablated, fish swam at greater distances from their neighbors, spent less time in the school, swam faster with higher tail beat frequencies, but when they were in the school, mostly maintained synchrony with their nearest neighbors. In other words, fish with the anterior lateral line ablated tended to have higher tail beat frequencies on average than control fish or those with a complete ablation (Fig. 5B). This happened because they tended to lose the school and use higher tail beat frequencies to rejoin the school (Fig. 4C). When they returned to the school, however, they were able to match frequency and synchronize their tails with their neighbors (Fig. 5C). Giant danios with their posterior lateral line system ablated showed a different pattern. While they also swam at greater distances to their neighbors and spent less time in the school, they had higher tail beat frequencies than control or completely ablated fish, and they could not maintain their synchrony with their nearest neighbor fish (Figs. 4D and 5B and C). Immediately after treatment, fish with the entire lateral line system ablated still swam at the same distance to their neighbors and the same tail beat frequency as controls, though they did spend less time in school. Even though their mean tail beat frequency was not significantly different from control fish, it varied substantially, which meant that they did not maintain synchrony with their nearest neighbor. Overall, the results provide evidence that the posterior lateral line is required for synchronization of tail beats, but the anterior lateral line allows for better matching of swimming speed within the school as it has a higher sensitivity to the local environment. The results suggest that the two regions of the lateral line system have different functions and thus are likely tuned for these different functions.
In conclusion, when the lateral line system was partially ablated, we observed that treated fish regularly lost track of their position in the school, which may have been caused by conflicting information between the visual and lateral line systems or by unreliable sensory inputs from a semi-intact lateral line system. During this period, the tail beat frequency of the treated fish increased, but fish could still synchronize their tails with their neighbors as long as the posterior lateral line was intact. Once the posterior lateral line was inactivated, phase locking of tail beats with the nearest neighbors decreased. In contrast, if the anterior lateral line is ablated, fish maintain tail-beat synchrony when they manage to stay within the school, but have trouble matching velocity within the school. Thus, our results indicate that the two branches of the lateral line system have different functions.
Data availability
Processed dataset is available from LabArchives (http://dx.doi.org/10.25833/va3k-nd56).
Author contributions
P.J.M., M.A.B.S., and E.D.T.: conceptualization, methodology, visualization, and writing—review and editing. P.J.M.: writing—original draft preparation and investigation. P.J.M. and E.D.T.: software, validation, and funding acquisition. P.J.M. and F.N.: formal analysis and data processing. E.D.T.: supervision and resources.
Supplementary Material
Acknowledgments
The authors thank Annie Phan for tracking videos of schooling fish; the laboratory members of the Tytell Lab for valuable insights and feedback during the initial stages of the project and animal husbandry; Dr. Ulrike K. Müller and Suzanne Miller for the technical assistance in the submission of this manuscript; and, finally, Janneke M. Schwaner for the S5 Symposium invitation to submit a talk and manuscript to the Society for Integrative and Comparative Biology conference that took place on January 2021 in Washington DC.
Funding
This study was supported in part by the National Science Foundation under grant RCN-PLS 1062052 (to Dr. Lisa J. Fauci and Avis H. Cohen), grant IOS 1652582 (to E.D.T.), the Soft Material Robotics Integrative Graduate Education and Research Traineeship (IGERT) program under grant IGERT-1144591 (to Dr. Barry A. Trimmer and Dr. David Kaplan), and the National Institute of Health Training in Education and Critical Research Skills under grant K12GM074869 (to M.A.B.S).
From the symposium “An evolutionary tail: Evo-Devo, structure, and function of post-anal appendages” presented at the virtual annual meeting of the Society for Integrative and Comparative Biology, January 3–7, 2021.
References
- Ashraf I, Bradshaw H, Ha T-T, Halloy J, Godoy-Diana R, Thiria B.. 2017. Simple phalanx pattern leads to energy saving in cohesive fish schooling. Proc Natl Acad Sci USA 114:201706503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf I, Godoy-Diana R, Halloy J, Collignon B, Thiria B.. 2016. Synchronization and collective swimming patterns in fish (Hemigrammus bleheri). J R Soc Interface 13:20160734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge R. 1958. The speed of swimming of fish as related to size and to the frequency and amplitude of the tail beat. J Exp Biol 35:109–33. [Google Scholar]
- Batschelet E. 1981. Circular statistics in biology. In: Sibson R, Cohen JE, editors. Mathematics in biology. London: Academic Press. [Google Scholar]
- Butler JM, Field KE, Maruska KP.. 2016. Cobalt chloride treatment used to ablate the lateral line system also impairs the olfactory system in three freshwater fishes. PLoS ONE 11:e0159521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal EA, Radford CA, Mensinger AF.. 2018. The potential for the anterior lateral line to function for sound localization in toadfish (Opsanus tau). J Exp Biol 221:jeb180679. [DOI] [PubMed] [Google Scholar]
- Carrillo A, McHenry MJ.. 2016. Zebrafish learn to forage in the dark. J Exp Biol 219:582–9. [DOI] [PubMed] [Google Scholar]
- Carrillo A, Van Le D, Byron M, Jiang H, McHenry MJ.. 2019. Canal neuromasts enhance foraging in zebrafish (Danio rerio). Bioinspir Biomim 14:035003. [DOI] [PubMed] [Google Scholar]
- Chicoli A, Butail S, Lun Y, Bak-Coleman J, Coombs S, Paley DA.. 2014. The effects of flow on schooling Devario aequipinnatus: school structure, startle response and information transmission. J Fish Biol 84:1401–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs S, Bleckmann H, Fay RR, Popper ANeditors. 2014. The lateral line system. New York (NY): Springer. [Google Scholar]
- Coombs S, Montgomery J.. 2014. The role of flow and the lateral line in the multisensory guidance of orienting behaviors. In: Flow sensing in air and water: behavioral, neural and engineering principles of operation. Heidelberg, New York, Dordrecht, London: Springer. p. 65–102. [Google Scholar]
- Coombs S, Van Netten S.. 2005. The hydrodynamics and structural mechanics of the lateral line system. Fish Physiol 23:103–39. [Google Scholar]
- Coombs S, Bak-Coleman J, Montgomery J.. 2020. Rheotaxis revisited: a multi-behavioral and multisensory perspective on how fish orient to flow. J Exp Biol 223:jeb223008. [DOI] [PubMed] [Google Scholar]
- Cruz IA, Kappedal R, Mackenzie SM, Hailey DW, Hoffman TL, Schilling TF, Raible DW.. 2015. Robust regeneration of adult zebrafish lateral line hair cells reflects continued precursor pool maintenance. Dev Biol 402:229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daghooghi M, Borazjani I.. 2016. The hydrodynamic advantages of synchronized swimming in a rectangular pattern. Bioinspir Biomim 10:1–23. [DOI] [PubMed] [Google Scholar]
- Denton EJ, Gray JAB.. 1988. Mechanical factors in the excitation of the lateral lines of fishes. Sens Biol Aquat Anim 2:595–617. [Google Scholar]
- Dunnett CW. 1955. A Multiple Comparison Procedure for Comparing Several Treatments with a Control. J Am Stat Assoc 50:1096–121. [Google Scholar]
- Faucher K, Parmentier E, Becco C, Vandewalle N, Vandewalle P.. 2010. Fish lateral system is required for accurate control of shoaling behaviour. Anim Behav 79:679–87. [Google Scholar]
- Fisher NI. 1993. Statistical analysis of circular data [Database]. Cambridge: Cambridge University Press. [Google Scholar]
- Harris JA, Cheng AG, Cunningham LL, MacDonald G, Raible DW, Rubel EW.. 2003. Neomycin-induced hair cell death and rapid regeneration in the lateral line of zebrafish (Danio rerio). J Assoc Res Otolaryngol 4:219–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick TL. 2008. Software techniques for two- and three-dimensional kinematic measurements of biological and biomimetic systems. Bioinspir Biomim 3:034001. [DOI] [PubMed] [Google Scholar]
- Herskin J, Steffensen JF.. 1998. Energy savings in sea bass swimming in a school: measurements of tail beat frequency and oxygen consumption at different swimming speeds. J Fish Biol 53:366–76. [Google Scholar]
- Inada Y, Kawachi K.. 2002. Order and flexibility in the motion of fish schools. J Theor Biol 214:371–87. [DOI] [PubMed] [Google Scholar]
- Kalmijn AJ. 1988. Hydrodynamic and acoustic field detection. In: Atema J, Fay RR, Popper AN, Tavolga WN, editors. Sensory biology of aquatic animals. New York (NY): Springer. p. 83–130. [Google Scholar]
- Killen SS, Marras S, Steffensen JF, McKenzie DJ.. 2012. Aerobic capacity influences the spatial position of individuals within fish schools. Proc R Soc B Biol Sci 279:357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroese ABA, Schellart NAMM.. 1987. Velocity- and acceleration-sensitive units in the trunk lateral line of the trout. J Physiol 68:2212–21. [DOI] [PubMed] [Google Scholar]
- Magrassi L, Purves D, Lichtman JW.. 1987. Fluorescent probes that stain living nerve terminals. J Neurosci 7:1207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marras S, Killen SS, Lindström J, McKenzie DJ, Steffensen JF, Domenici P.. 2015. Fish swimming in schools save energy regardless of their spatial position. Behav Ecol Sociobiol 69:219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee A, Soto AP, Chen P, McHenry MJ.. 2020. The sensory basis of schooling by intermittent swimming in the rummy-nose tetra (Hemigrammus rhodostomus). Proc Biol Sci 287:20200568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekdara PJ, Schwalbe MAB, Coughlin LL, Tytell ED.. 2018. The effects of lateral line ablation and regeneration in schooling giant danios. J Exp Biol 221:jeb175166. [DOI] [PubMed] [Google Scholar]
- Middlemiss KL, Cook DG, Jerrett AR, Davison W.. 2017. Morphology and hydro-sensory role of superficial neuromasts in schooling behaviour of yellow-eyed mullet (Aldrichetta forsteri). J Comp Physiol A 203:807–17. [DOI] [PubMed] [Google Scholar]
- Nair A, Changsing K, Stewart WJ, McHenry MJ.. 2017. Fish prey change strategy with the direction of a threat. Proc R Soc B 284:20170393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novati G, Verma S, Alexeev D, Rossinelli D, van Rees WM, Koumoutsakos P.. 2017. Synchronisation through learning for two self-propelled swimmers. Bioinspir Biomim 12:036001. [DOI] [PubMed] [Google Scholar]
- Olsen EM, Vøllestad LA.. 2001. An evaluation of visible implant elastomer for marking age-0 brown trout. North Am J Fish Manag 21:967–70. [Google Scholar]
- Oteiza P, Odstrcil I, Lauder G, Portugues R, Engert F.. 2017. A novel mechanism for mechanosensory-based rheotaxis in larval zebrafish. Nature 547:445–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge BL, Pitcher TJ.. 1980. The sensory basis of fish schools: relative roles of lateral line and vision. J Comp Physiol A 135:315–25. [Google Scholar]
- Pinto-Teixeira F, Viader-Llargués O, Torres-Mejía E, Turan M, González-Gualda E, Pola-Morell L, López-Schier H.. 2015. Inexhaustible hair-cell regeneration in young and aged zebrafish. Biol Open 4:903–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher TJ, Partridge BL, Wardle CS.. 1976. A blind fish can school. Science 194:963–5. [DOI] [PubMed] [Google Scholar]
- Ristroph L, Liao JC, Zhang J.. 2015. Lateral line layout correlates with the differential hydrodynamic pressure on swimming fish. Phys Rev Lett 114:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth E, Hall RW, Daniel TL, Sponberg S.. 2016. Integration of parallel mechanosensory and visual pathways resolved through sensory conflict. Proc Natl Acad Sci USA 113:12832–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos F, MacDonald G, Rubel EW, Raible DW.. 2006. Lateral line hair cell maturation is a determinant of aminoglycoside susceptibility in zebrafish (Danio rerio). Hear Res 213:25–33. [DOI] [PubMed] [Google Scholar]
- Schwalbe MAB, Webb JF.. 2014. Sensory basis for detection of benthic prey in two Lake Malawi cichlids. Zoology 117:112–21. [DOI] [PubMed] [Google Scholar]
- Schwalbe MAB, Bassett DK, Webb JF.. 2012. Feeding in the dark: lateral-line-mediated prey detection in the peacock cichlid Aulonocara stuartgranti. J Exp Biol 215:2060–71. [DOI] [PubMed] [Google Scholar]
- Schwalbe MAB, Sevey BJ, Webb JF.. 2016. Detection of artificial water flows by the lateral line system of a benthic feeding cichlid fish. J Exp Biol 219:1050–9. [DOI] [PubMed] [Google Scholar]
- Song J, Yan HY, Popper AN.. 1995. Damage and recovery of hair cells in fish canal (but not superficial) neuromasts after gentamicin exposure. Hear Res 91:63–71. [DOI] [PubMed] [Google Scholar]
- Takagi T, Ito S, Torisawa S, Inada Y.. 2013. Energy-saving Effect of Fish Schooling in the Japanese Mackerel, Scomber japonicus. Math Phys Fish Sci 10:2–13. [Google Scholar]
- Theriault DH, Fuller NW, Jackson BE, Bluhm E, Evangelista D, Wu Z, Betke M, Hedrick TL.. 2014. A protocol and calibration method for accurate multi-camera field videography. J Exp Biol 217:1843–8. [DOI] [PubMed] [Google Scholar]
- van Netten SM, Kroese ABA.. 1987. Laser interferometric measurements on the dynamic behaviour of the cupula in the fish lateral line. Hear Res 29:55–61. [DOI] [PubMed] [Google Scholar]
- Van Trump WJ, Coombs S, Duncan K, McHenry MJ.. 2010. Gentamicin is ototoxic to all hair cells in the fish lateral line system. Hear Res 261:42–50. [DOI] [PubMed] [Google Scholar]
- Verma S, Novati G, Koumoutsakos P. (2018). Efficient collective swimming by harnessing vortices through deep reinforcement learning. PNAS 115: 5849–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb JF, Bird NC, Carter L, Dickson J.. 2014. Comparative development and evolution of two lateral line phenotypes in lake malawi cichlids. J Morphol 275:678–92. [DOI] [PubMed] [Google Scholar]
- Weihs D. 1973. Hydromechanics of fish schooling. Nature 241:290–1. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Processed dataset is available from LabArchives (http://dx.doi.org/10.25833/va3k-nd56).