Figure 4.
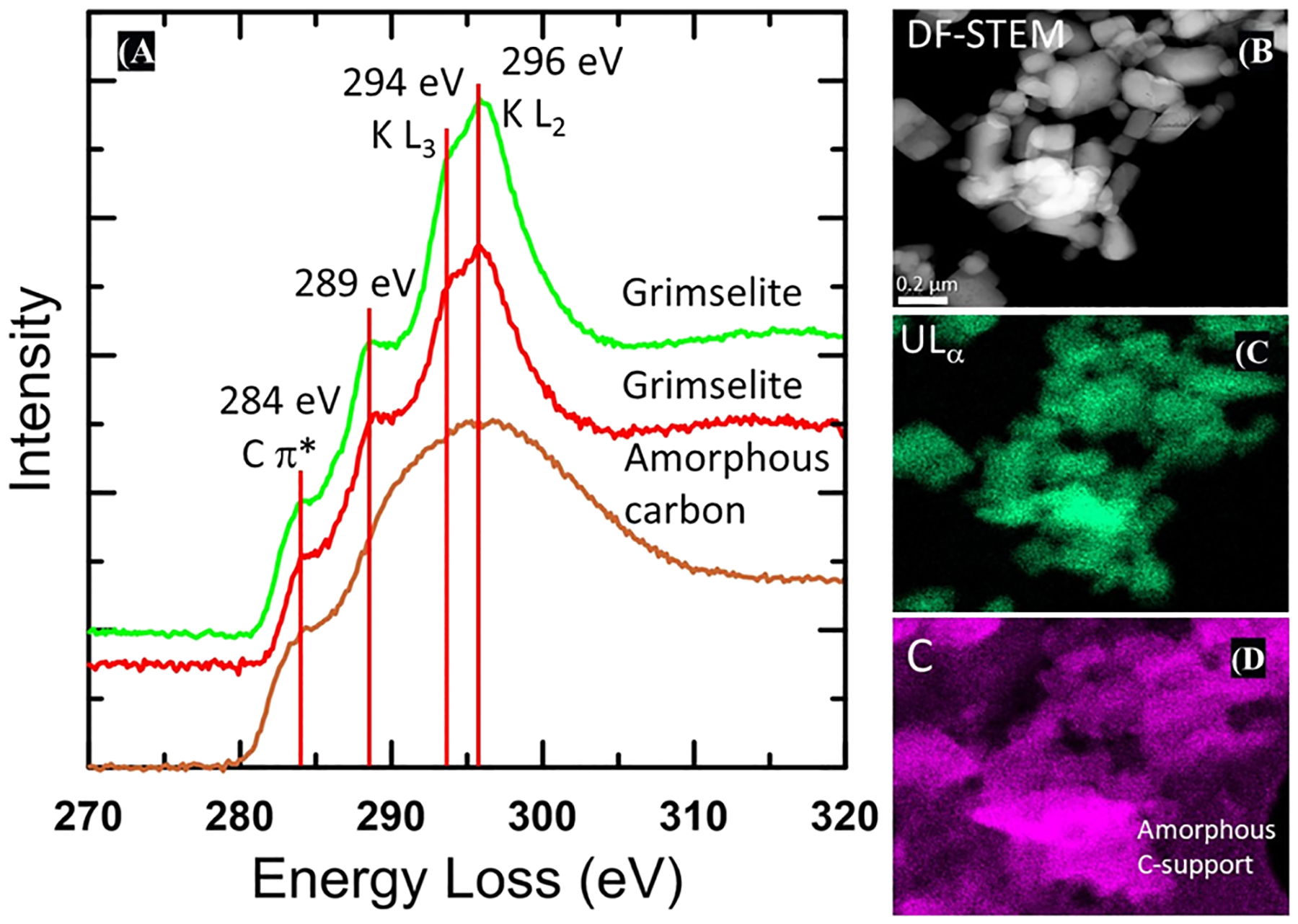
EELS from solid collected after the reaction of U–KCl-NOM at pH 4 for 24 h. (A) DF-STEM image (B) and STEM–EDS X-ray maps (C,D). Two EEL spectra for grimselite are for the crystalline U- and K-bearing solids, which have been identified as grimselite-based electron diffraction data (Figure 3) are shown in red and green showing the presence of the K L3 and L2 3 edges at 294 and 296 eV, respectively. A distinct shoulder is present on the lower energy side of the K edge at 289 eV, which could be attributable to either carbonate or carboxylic groups. The lower spectrum (brown) is from the amorphous holey carbon film support with a distinct 284 eV edge that can be assigned to the C π* peak. This feature is also apparent in the grimselite spectra because the crystallites occur directly on the holey carbon film support. The 289 eV feature is not present in the amorphous carbon substrate. Right hand images show a DF-STEM image of the crystallites and X-ray maps of U and C, demonstrating that the crystallites contain C associated with U. An X-ray signal from the amorphous holey carbon film is clearly apparent in the lower right and upper left of the carbon X-ray map.
