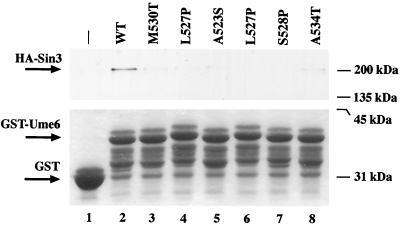
FIG. 5.
GST pulldown assay. HA-Sin3 from yeast extracts was bound to GST (lane 1) or GST fused to amino acids 508 to 584 of wild-type (wt; lane 2) or mutant (lanes 3 to 8) Ume6 as described in Materials and Methods. A single HA-Sin3 lysate was evenly divided among the tubes (500 μg each). Complexes were eluted with boiling loading buffer, separated by SDS-polyacrylamide gel electrophoresis and visualized with anti-HA antibodies (top) to assay Sin3 interaction or Coomassie (bottom) to verify equivalent amounts of GST-Ume6 fusion in each sample. Several presumed GST-Ume6 degradation products uniformly appear (as previously observed [55]) in all of the GST-Ume6 lanes.