FIGURE 4.
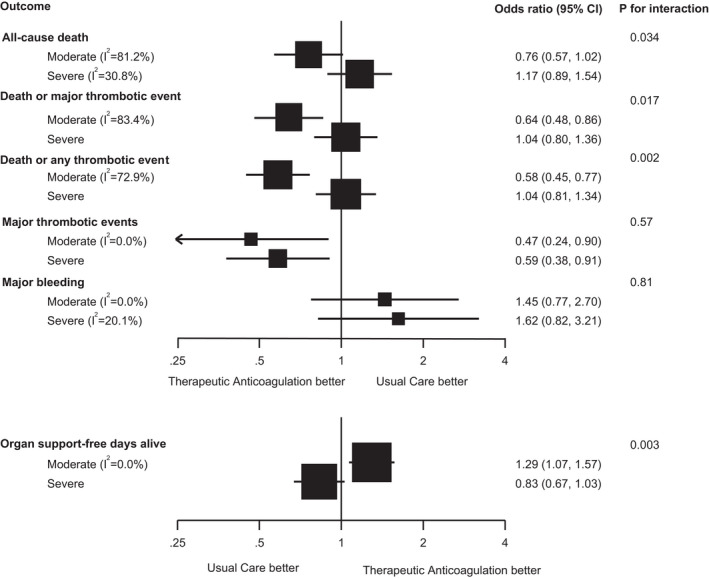
Analyses of the interaction between treatment effect and severity of illness of therapeutic heparin versus usual care in patients with COVID‐19. The analysis is based on Mantel‐Haenszel fixed‐effect meta‐analyses of the RAPID trial, HEP‐COVID trial, and the multiplatform trial in moderately ill ward patients, 9 , 21 , 22 and results of the multiplatform trial, HESACOVID, and HEP‐COVID trial in severely ill ICU patients. 10 , 22 , 24 Squares and horizontal lines show treatment effects and their 95% confidence intervals in each subgroup. The area of each square is proportional to the inverse of the variance in the subgroup. Odds ratios for organ support‐free days alive are from ordinal logistic regression in all trials; death up to 28 days was assigned the worst outcome (a value of −1) in all trials. The P values for interaction are for the comparison of treatment effects between moderately and severely ill patients and were derived from a chi‐squared test. Major thrombotic events were defined as the composite of myocardial infarction, pulmonary embolism, ischemic stroke, or systemic arterial embolism; any thrombotic events were defined as a major thrombotic event or deep vein thrombosis; major bleeding defined by the ISTH Scientific and Standardization Committee. 15 The observation time for the outcomes in the trials were 28 days for the multiplatform trials (with the exception of organ support free days which was calculated for an observation time of 21 days), 28 days for the RAPID trial and 30 days for the HEP‐COVID trial 16 , 17 , 23
