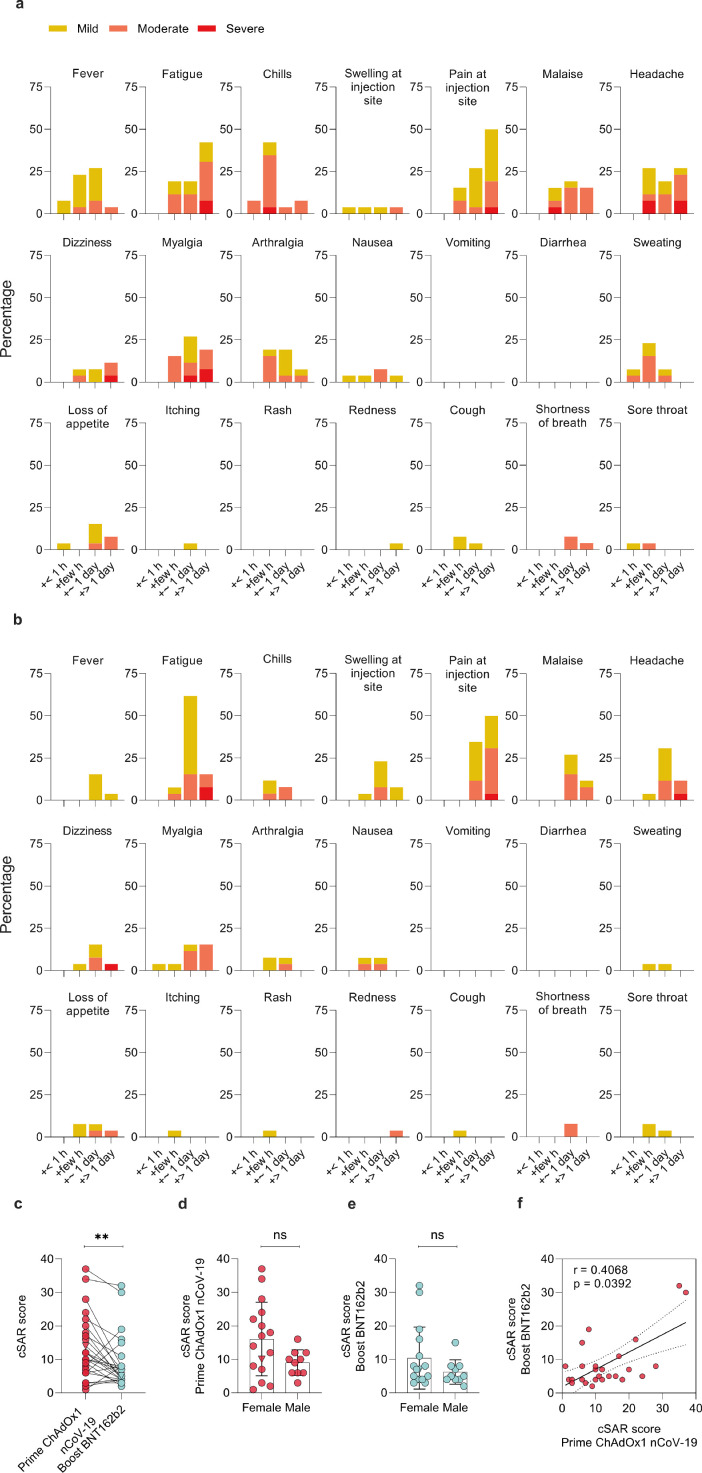
Figure 1.
Solicited adverse reactions following ChAdOx1 nCoV-19 prime and BNT162b2 boost vaccination. Percentages of n=26 participants with individual symptoms following prime (a) or boost (b) vaccination. Severity is graded on a scale of 1-2 (for some symptoms) or 1-3 (for most), as adapted from the Common Terminology Criteria for Adverse Events (US Department of Health and Human Services, Version 4.03).25 (c) Cumulative solicited adverse reaction (cSAR) scores of all participants following prime and boost vaccination. For calculation of cSAR scores, symptom gradings are summed and an additional score point is added for symptoms lasting more than 24 h. Paired two-tailed t-test compares prime and boost vaccination. (d,e) Analysis of cSAR scores by participant sex (Mann-Whitney-U test). (f) Spearman correlation of cSAR scores following prime and boost vaccination. The SARS-CoV-2 convalescent individual (triangle) was excluded in all statistical analyses.; ns not significant; ** p < 0.01