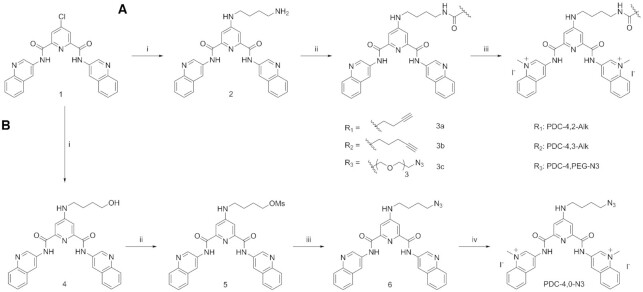
Scheme 1.
Synthesis of copper-catalyzed alkyne-azide cycloaddition (CuAAC) PDC precursors: PDC-4,2-Alk, PDC-4,3-Alk and PDC-4,PEG-N3. Route A reagents and conditions: (i) 1,4-diaminobutane (100 equiv), NEt3 (5 equiv), 95°C, 16 h, 90%; (ii) Carboxylic acid (1.5 equiv), EDCI (1.5 equiv), HOBt (0.15 equiv), DMF, rt, 16 h; R1= 4-pentynoic acid, 77%; R2 = 5-hexynoic acid, 70%; R3 = 11-azido-3,6,9-trioxaundecanoic acid, 61%; (iii) CH3I (235 equiv), DMF, 40°C, 16 h, R1 = 95%; R2 = 93%; R3 = 76%. DMF = N,N-dimethylformamide; EDCI = 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide; HOBt = Hydroxybenzotriazole. Route B reagents and conditions: (i) 4-amino-1-butanol (18.7 equiv), NEt3 (2.7 equiv), 90°C, 16 h, 66%; (ii) MsCl (4.5 equiv), NEt3 (9.0 equiv), DCM, 0°C to rt, 1 h 30 min, 67%; (iii) NaN3 (10 equiv), DMF, 110°C, 2 h, 77%; (iv) CH3I (250 equiv), DMF, 40°C, 16 h, 87%. MsCl = Methanesulfonyl chloride.