Fig. 3. Molecular changes induced by PTH7d.
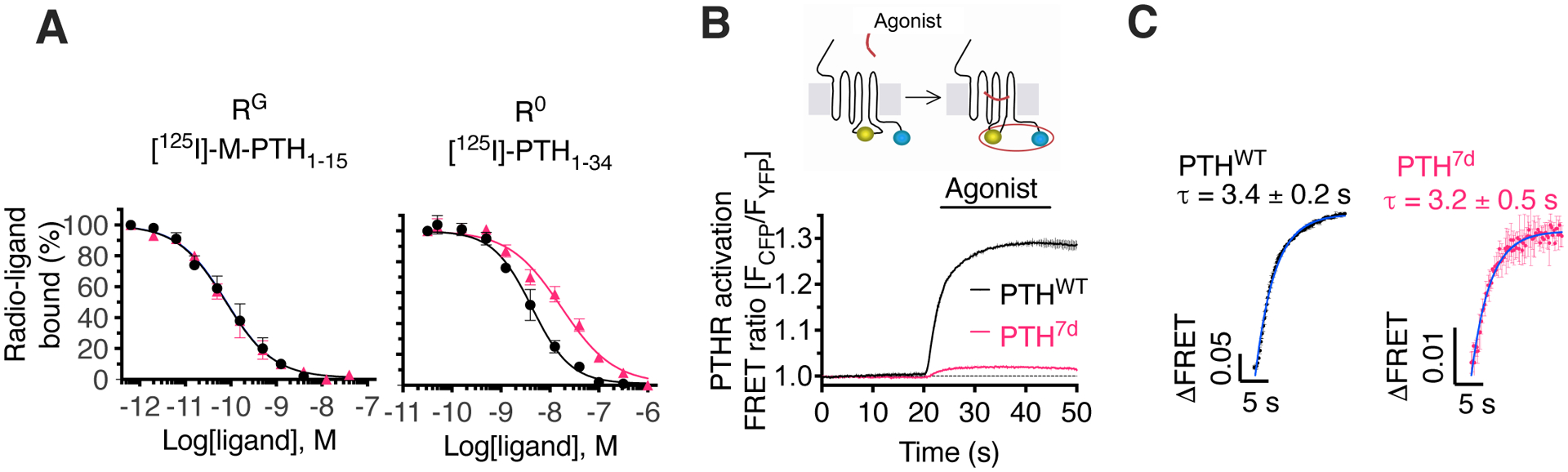
(A) Competition binding at equilibrium with 125I-PTH1–15 and 125I-PTH1–34 as radioligands to detect the RG and R0 states of PTHR, respectively. Data are means ± SEM from N = 5 independent experiments with duplicate wells for each concentration.
(B and C) The inset shows a schematic of the FRET-based PTHR activation sensor (PTHRCFP/YFP) with YFP (yellow) fused to ICL3, and CFP (blue) attached to the receptor C-terminal tail. The graph shows averaged time-courses of PTHR activation by recording changes of the FRET ratio in HEK 293 cells expressing PTHRCFP/YFP with the initial value at t = 0 set to 1 (B), and kinetics of PTHR activation (C). Cells were continuously perfused with control buffer or 1 μM agonist (horizontal bar). Data are means ± SD from N = 2 independent experiments. Note that the FRET data are expressed as FCFP/FYFP, resulting in a positive change upon agonist stimulation.
