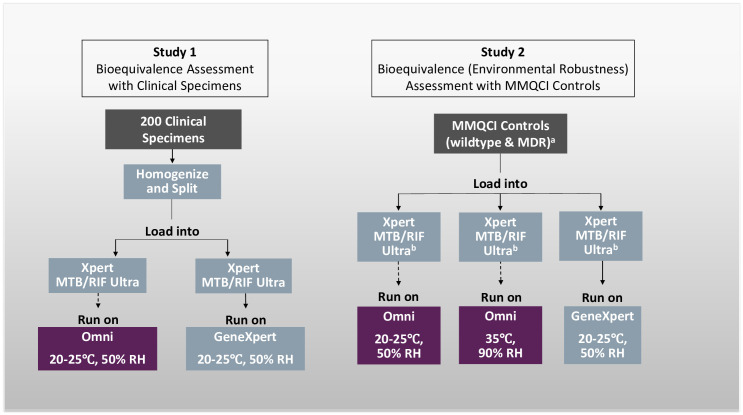
Fig 1. Omni bioequivalence study flow.
RH: relative humidity, MDR: multidrug-resistant tuberculosis, MMQCI: Maine Molecular Quality Controls, SR: sample reagent. a 30 replicates of each control were tested by each device at the listed conditions (i.e. 30 replicates per condition) at 3x the limit of detection. Replicate testing was divided between 8 Omni devices and 6 modules of two GeneXpert-IV devices. Daily negative control testing was also performed for Omni and GeneXpert. b Ultra cartridges were stored at the respective condition (20–25°C, 50% RH or 35C, 90% RH) for 24hr prior to testing.