Implications.
Epigenetic factors respond to environmental factors and underlying genotypes to influence livestock phenotypic expression (e.g., milk yield, wool quality, disease resistance, growth, and development, etc.).
Epigenetic alterations are associated with livestock traits and with differences in livestock product yields.
A window is opened for exploring the mechanisms of disease resistance in farm animals through epigenetic processes.
Consideration of genetic mechanisms of Mendelian inheritance and epigenetic mechanisms of non-Mendelian inheritance may further increase genetic gain in livestock trait improvement.
Epigenetic factors provide additional levels of livestock trait regulation and are relevant for livestock management and improvement.
Introduction
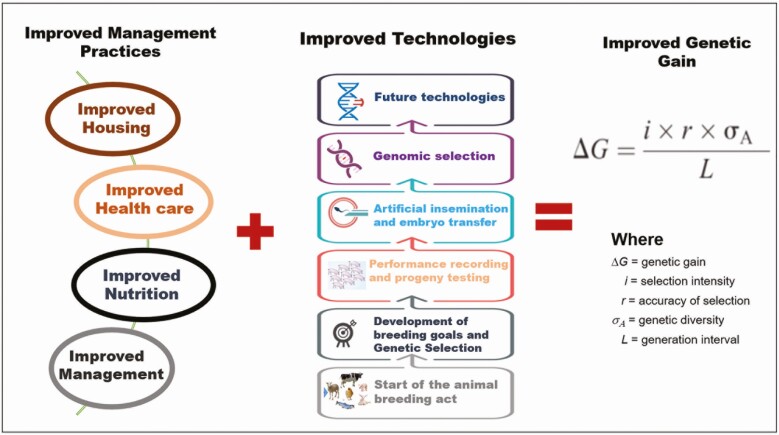
Over the years, the implementation of selective breeding and other improved management practices led to increased livestock productivity. In particular, improved practices over the last 80 years resulted in tremendous improvement in animal production; for example, the average milk produced per Holstein cow has more than doubled and feed efficiency in broiler chickens allow a 4-fold increase in body size with the same amount of feed input. In general, improved livestock productivity, for example, relies on increasing the rate of genetic gain (ΔG); and breeding programs will adopt improved practices (e.g., improved nutrition, improved management practices, improved health care, genetic selection, reproduction technologies, genomic selection, etc.) that increase the intensity of selection, selection accuracy, and genetic diversity while decreasing the generation interval (Figure 1). Moreover, the implementation of genomic breeding, which relies on the use of genomic information in the form of single nucleotide polymorphisms (SNP) resulted in rapid genetic improvement in lowly heritable traits (e.g., fertility, lifespan, and health traits, etc.), shortened generation interval, selection of animals at an early age, higher rate of genetic gain, increased reliability of predicting breeding value and higher intensity of selection (Wiggans et al., 2017; Van Doormaal, 2019). However, the human population is increasing and with it, increasing market demand for animal products. To meet this demand, current practices must be tailored to enhance productivity, which requires knowledge of further processes that drive phenotypic expression and animal productivity. While genomic breeding relies on using SNP data to calculate genomic breeding values, it is important to note that genetic variation between individuals only accounts for a portion of the genomic variation and does not sufficiently explain the phenotype diversity (Langevin and Kelsey, 2013; Ibeagha-Awemu and Khatib, 2017). Moreover, the DNA sequence cannot illustrate the functional and morphological diversity and differentiation between cell populations in multicellular organisms nor capture the effects of epigenomics factors that respond to specific environmental circumstances and growth stages to influence phenotypic expression.
Figure 1.
Farm management practices and technologies that enhance genetic gain in livestock breeding and improvement.
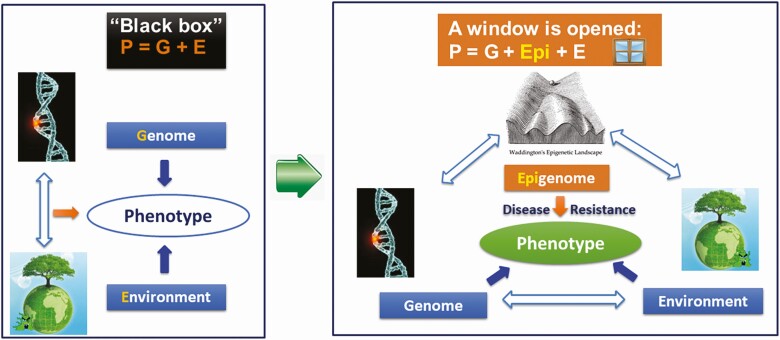
Looking back, traditional animal breeding practices generally considered that phenotypic value is equal to genotype value plus environmental effects (Figure 2, left panel). But in fact, the genotype and the environment values constitute a black box, because the extent of their interaction and actual effects on the phenotype is only beginning to emerge. With developments in the subject area of epigenetics and the discovery of epigenetic modifications, a “window” in this “black box” has been opened (Figure 2, right panel). Therefore, processes embedded in the epigenome provide additional avenues to investigate the secrets underlying phenotype diversity, and how they can be harnessed for improved productivity. The epigenome, which includes processes like DNA methylation, histone modification, chromatin remodeling, and non-coding RNA (ncRNA) regulation, regulate gene expression and therefore constitute significant players in modulating genome function and stability (Bird, 2002; Kouzarides, 2007; Morris and Mattick, 2014). Interestingly, these epigenetic factors have been shown to regulate livestock traits (Ibeagha-Awemu and Zhao, 2015; Do and Ibeagha-Awemu, 2017; Do et al., 2021; Wang and Ibeagha-Awemu, 2021) and could therefore be responsible for additional variation needed for continued improvements in livestock health and productivity.
Figure 2.
A window is opened for further exploration of the mechanisms of disease resistance in farm animals through epigenetic processes.
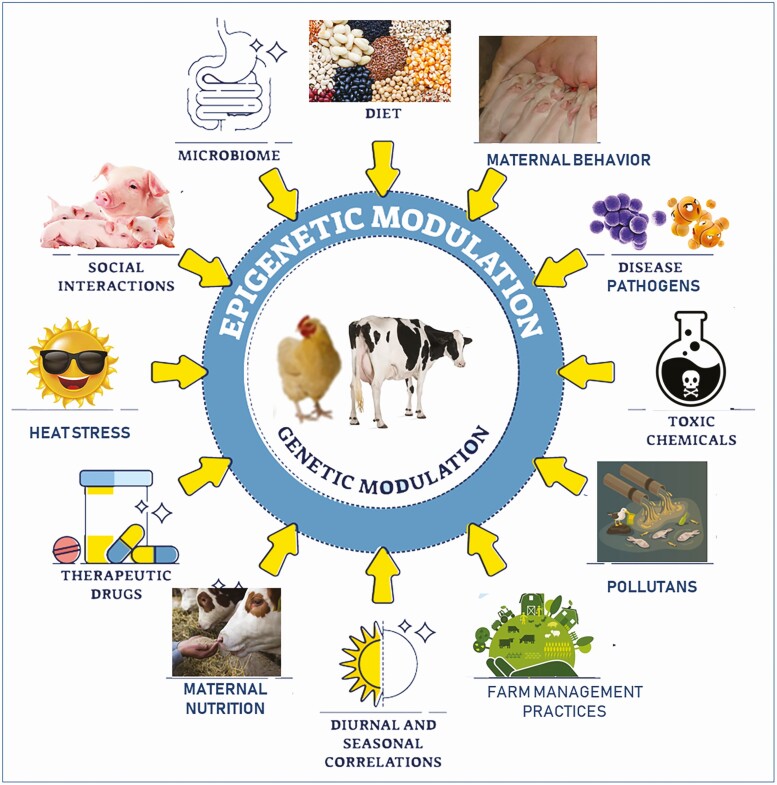
Epigenetics generally refers to mitotically stable states and molecular modifications related to gene activity without changing the DNA sequence. In other words, epigenetics describes the mechanisms underlying the phenotypic changes rather than DNA sequence alterations, thereby providing an alternative layer of information. Moreover, epigenetic mechanisms act as intermediates between environmental factors and host genotype thereby contributing to the regulation of various cellular processes and the expression of the phenotype (Figure 3). Furthermore, epigenetic alterations are specific to specific life stages or specific organs, and vary under various conditions and are therefore dynamic throughout a lifetime. Notably, embryogenesis is the peak time of epigenetic reprogramming, specially DNA methylation, but throughout growth and development, epigenetic factors respond to various environmental cues (the exposome) and the underlying genotype to continually modify cellular activity and phenotypic expression (Figure 3). During a production cycle, livestock goes through various stages of development, are subjected to various management practices and exposed to many factors (e.g., pathogens, nutrition, environmental stressors, etc.); therefore, understanding the role of epigenetics will open a window (Figure 2, right panel) to quantify a part of the missing causality and heritability of complex livestock health and production traits. Data from our work and other colleagues on the relevance of how these additional levels of regulators contribute to phenotypic expression of livestock traits, and how they can be exploited for improved livestock productivity and health management will be discussed in subsequent sections. In particular, postnatal epigenetic reprogramming will be discussed.
Figure 3.
Epigenetic factors respond to external and internal environment factors (the exposome) and interact with the underlying genotype to influence phenotypic outcomes.
Do Epigenetic Processes Influence Livestock Productivity and Growth?
Available data indicate that epigenetic processes respond to various stimuli including nutrition, pathogens, maternal and paternal behavior or stress, environmental stressors, management practices, chemicals, and pollutants to induce epigenetic alterations causing changes in phenotypic expression in livestock animals (Figure 3; Wang and Ibeagha-Awemu, 2021). In this section, some of the evidence of the effects of these epigenetic factors on livestock phenotypic expression will be examined.
Nutritional stimuli and environmental perturbations
In farm animal production, nutrition is among the most import cost that determines farm profitability. Following conception, nutrition is the foremost environmental exposome that interacts with an individual’s genome to determine its growth, development, and phenotypic expression. Adequate nutrition and type of nutrients fed to animals are of vital importance, and they have been shown to influence the epigenome. Mounting evidence indicates that alterations of a permanent nature in the epigenome of embryos or germline could be transferred to offspring, a phenomenon referred to as intergeneration or transgenerational epigenetic inheritance (Miska and Ferguson-Smith, 2016; Perez and Lehner, 2019).
In recent times, consistent nutritional stimulus has been shown to cause epigenetic modifications in somatic tissues and their effects on health and diseases of livestock could be transmitted between generations (Xue et al., 2016; Ideraabdullah and Zeisel, 2018). Moreover, excessive lack of nutrition or excessive availability of nutrients are also known to alter the epigenetic state of germ cells and transmission to subsequent generations (Guo et al., 2020). A plethora of investigations indicate that nutritional stimulus with different categories of nutrients induced epigenetic modifications resulting in altered gene and phenotypic expression (some of the evidence is summarized in Table 1). These pieces of evidence indicate that nutrition-induced epigenetic alterations can be heritable, but the underlying mechanisms are not clear. What is clear, is that DNA methylation modifications in response to nutritional stimulus certainly cause alteration in production performance and possibly disease susceptibility.
Table 1.
Examples of nutritional stimulus impact on epigenetic alterations in some livestock species
| Dietary treatment | Epigenetic changes | References |
|---|---|---|
| Methionine supplementation of Holstein cows throughout the periparturient period | Global DNA methylation was lower while promoter methylation of PPARA gene was higher in liver tissues | Osorio et al., 2016 |
| Grass-fed and grain-fed Angus cattle | A total of 217 DMR including in ADAMTS3 and ENPP3 genes were identified in rumen tissues | Li et al., 2019 |
| Holstein cows fed high- vs. low-concentrate diets | Chromatin loosening at the promoter regions of TLR4, LBP, HP, SAA3 genes in the liver | Chang et al., 2015 |
| Feed restriction (85% vs. 140%) of Angus-Simmental cross-bred cow diets | One DMR in IGF2 gene in longissimus dorsi and semitendinosus muscles | Paradis et al., 2017 |
| Vitamin C supplementation of pig’s diet | Methylation erasure on 5mC, m6A, and H3K27me3, but the establishment of H3K4me3 and H3K36me3 in oocytes. Examples of affected genes: TET2, DNMT3B, HIF-1α, HIF-2α, TET3, METTL14, KDM5b, and EED | Yu et al., 2018 |
| Supplementation of piglet’s diet with omega-3 fatty acid | One hypomethylated DMR in chromosome 4, 2 hypermethylated DMRs in chromosomes 4 and 12 in leucocytes. Example of affected gene: RUNX1T1 | Boddicker et al., 2016 |
| Maternal methyl donors dietary supplementation of Large White × Landraces sows | Increased methylation at the promoter of IGF-1 in methyl donor group in Liver samples from piglets | Jin et al., 2018 |
| Maternal genistein supplementation of the diets of Laying broiler breeder hens | Induced H3K36me3 and H4K12ac at the PPARD promoter in liver samples from pullets | Lv et al., 2019 |
| Prenatal betaine supplementation of the diets of Juvenile chicken | Increased CpG methylation at the promoter regions of genes (e.g., LXR and CYP27A1a) in liver samples from juvenile chickens | Hu et al., 2017 |
| Maternal betaine supplementation of the diets of Rugao yellow-feathered laying hens | Hypomethylated promoter regions of steroidogenic genes (e.g., AHCYL, GNMT1, and BHMT) in blood from pullets | Abobaker et al., 2019 |
DMR, differentially methylated region; DMC, differentially methylated cytosine; H3K27me3, the tri-methylation at the 27th lysine residue of the histone H3 protein; H3K4me3, the tri-methylation at the 4th lysine residue of the histone H3 protein; H3K36me3, the tri-methylation at the 36th lysine residue of the histone H3 protein; H4K12ac, the acetylation at the 12th lysine residue of the histone H4 protein.
For specific examples, supplementation of the diets of cows with methionine throughout the periparturient period resulted in lowered global DNA methylation state of the liver, higher promoter methylation of PPARA (peroxisome proliferator-activated receptor alpha) gene (a nuclear receptor for long-chain fatty acids) and increased expression of PPARA gene in supplemented cows compared to non-supplemented cows (Osorio et al., 2016). Moreover, the expression of the gene targets of PPARA (ANGPTL4 [angiopoietin-like 4], FGF21 [fibroblast growth factor 21], and PCK1 [phosphoenolpyruvate carboxykinase 1]) were also increased, indicating that supplemental methionine activated the PPARA-regulated signaling pathway. Activation or upregulation of the hepatic PPARA pathway is known to been associated with improved lipid metabolism and immune function. Furthermore, maternal methionine supply during late pregnancy programmed hepatic metabolism of calves by maintaining methionine homeostasis, DNA methylation, energy metabolism thereby possibly contributing to better nutrient utilization efficiency, growth promotion, and enhanced development performance (Alharthi et al., 2019). In other studies, dietary restriction, feeding high- vs. low-concentrate diets, grain vs. grass, mineral/vitamin, and fatty acid supplementation of the diets of pigs, cows, or chickens altered the epigenetic state of various tissues or cells resulting in various phenotypes (Table 1).
Environmental perturbations including heat stress, weaning stress, transportation stress, pathogens, dietary changes, and so on, limit livestock productivity through impacts on epigenetic factors, gene expression, metabolism, and immune response with direct effects on health, welfare, and productivity. For example, epigenetic mechanisms have been found to induce blood DNA methylation changes between heat-stressed and recovery periods in Nellore cattle (Del Corvo et al., 2021), or in various tissues (kidney, liver, muscle, lungs, heart, and brain) in response to hypoxic stress in Tibetan and Yorkshire pigs, Tibetan goat, Tibetan sheep, Chuanzhong goat, and small-tailed Han sheep (Wang et al., 2017b; Zhang et al., 2019). Moreover, stress due to transportation of Brahman cows induced epigenetic alterations in the blood of both cows and offspring (Littlejohn et al., 2018). Weaning, the process of separating young from their mothers, is a period of stress (e.g., dietary and social stress) linked to changes in the immune response and susceptibility to diseases in livestock production. Recently, weaning stress was found to induce DNA methylation and gene expression changes in weaned piglet peripheral blood mononuclear cells (Corbett et al., 2021).
Epigenetic alterations are associated with differences in livestock product yields
Livestock products of high economic importance like milk, beef, egg, and wool are under the regulation of epigenetic factors and other factors (e.g., genetics, nutrition, health, farm management, environmental factors, etc.).
Milk and its products.
In recent years, research has shown that epigenetic modifications have important regulatory functions in livestock dairy animals with important effects on milk and dairy products (Wang and Ibeagha-Awemu, 2021). Following the pioneer report that abnormal DNA methylation at the STAT5-binding enhancer of the CSN1S1 (αS1-casein) promoter undesirably regulated αS1-casein synthesis in milk during lactation (Vanselow et al., 2006; Nguyen et al., 2014), subsequent studies have found effects of altered DNA methylation, chromatin modifications and ncRNA regulation on milk production in livestock. For example, differences in DNA methylation levels in blood were found between high and low milk yielding lactating dairy cows (Dechow and Liu, 2018); and of several milk-related genes (e.g., PPARα [peroxisome proliferator-activated receptor alpha], RXRα [retinoid X receptor alpha], and NPY [neuropeptide Y]) in the mammary glands of lactating goats at dry and lactation periods (Zhang et al., 2017b). Examining microRNA (miRNA) expression, reports found that miRNAs regulate the bovine lactation curve or specific stages of lactation (Do et al., 2017). Moreover, increased methylation levels of some lipid-related genes (FASN [fatty acid synthase], SCD1 [stearoyl-CoA desaturase], PPARG [peroxisome proliferator-activated receptor gamma], and SREBF1 [sterol regulatory element-binding transcription factor 1]) and impaired fatty acid synthesis following inhibition of miR-145 expression in goat have been reported (Wang et al., 2017a).
Meat, wool, and egg.
DNA methylation and miRNA regulation are important epigenetic mechanisms involved in the regulation of muscle development (meat) and wool production.
Studies on the DNA methylation profiles of longissimus dorsi muscles from sheep found differentially methylated genes (e.g., ADIPOQ [adiponectin, C1Q, and collagen domain containing], ITGA2 [integrin subunit alpha 2], MYOG [myogenin], CCNA2 [cyclin A2], MAPT [microtubule-associated protein tau], NR4A1 [nuclear receptor subfamily 4 group A member 1], DLK1 [delta-like non-canonical Notch ligand 1], COL1A2 [collagen type I alpha 2 chain], and DIAPH1 [diaphanous-related formin 1]) with determinative roles in muscle function (Fan et al., 2020). Meanwhile, DNA methylation differences in several genes (ACAD11 [acyl-CoA dehydrogenase family member 11], FASN, FADS6 [fatty acid desaturase 6], CAPN15 [calpain 15], NAT9 [N-acetyltransferase 9], and SPDEF [SAM pointed domain-containing ETS transcription factor]) were associated with changes in muscle development and meat quality in beef cattle breeds (Chen et al., 2019). In chickens, the DNA methylation profiles of later laying-period hen and juvenile hens with differential intramuscular fat deposition and water-holding capacities are different, an indication that DNA methylation affects intramuscular fat deposition through regulation of the expression of key genes (e.g., ABCA1 [ATP-binding cassette subfamily A member 1], GSTT1L [glutathione S-transferase theta 1-like], and COL6A1 [collagen type VI alpha 1 chain]) involved in this process (Zhang et al., 2017a, 2020).
As the economic value of wool production increases in the goat industry, recent knowledge indicates that epigenetic processes regulate cashmere wool production. For example, the genetic stability of wool traits between generations of cashmere goats is related to the state of DNA methylation (Dai et al., 2019). Furthermore, DNA methylation is involved in the regulation of wool fiber development and transformation of fur with special characters (e.g., curly wool with beautiful white color or high-quality brush hair; Qiang et al., 2018; Xiao et al., 2019). Also, a recent study examining miRNA expression in seven stages of fetal development, associated altered miRNA expression profiles with the development of secondary hair follicles in cashmere goats (Han et al., 2020). Moreover, circRNA (circular RNA) expression is different between the skins of Liaoning cashmere goats (produces high fiber) and Inner Mongolia cashmere goats (produces high-quality cashmere fiber) during the anagen period (Zheng et al., 2020).
Further specific examples of epigenetic influences on milk production and component yields, muscle development, beef tenderness, meat quality, fat deposition, fatty acid composition, muscle metabolism, boar taint, egg production, cashmere fiber growth, and other traits, and so on, are listed in Table 2. Collectively, these data indicate important regulatory roles of DNA methylation alterations in lactation, milk and component yields, meat, egg, and wool traits.
Table 2.
Differing livestock product phenotypes have altered epigenetic profiles
| Livestock phenotype (affected production trait) | Epigenetic changes in specific organs | References |
|---|---|---|
| High vs. low milk yielding Holstein cows (milk and protein yield) | The DNA methylation rates in blood from the lower-yielding cows were significantly higher than in blood from the higher-yield animals | Wang et al., 2019b |
| Xinong Saanen goats at different lactation stages (milk production) | The DNA methylation levels of 95 and 54 genes in mammary gland tissue in the lactation period were up- or downregulated, respectively, relative to the dry period | Zhang et al., 2017b |
| Guanzhong goat fed high or low-concentrate diets (milk fat production and composition) | Increased DNA methylation level in the promoter regions of the ACACA and SCD genes in mammary gland tissue | Tian et al., 2017 |
| Japanese black and Chinese Red Steppes cattle with different meat-production abilities (muscle development and meat quality traits) | A total of 23,150 DMRs were identified including 331 DMRs which correlated negatively with the expression of the corresponding genes in longissimus dorsi muscles | Fang et al., 2017 |
| Angus beef of divergent tenderness (beef tenderness) | DNA methylation profiles related to beef tenderness, and identification of 7,215 DMRs in longissimus dorsi muscles between tender and tough beef | Zhao et al., 2020 |
| Simmental, Yunling, and Wenshan cattle breeds differing in meat-production abilities (meat quality) | 18 DMC reported in longissimus dorsi muscles between Simmental and Wenshan cattle, 14 DMC between Simmental and Yunling cattle and 28 DMC between Wenshan and Yunling cattle | Chen et al., 2019 |
| Obese [Rongchang] and lean [Landrace pigs] type pig breeds (fat deposition and fatty acid composition) | 483 DMRs identified in the promoter regions of several genes in back fat | Zhang et al., 2016 |
| Castrated and non-castrated Huainan pigs (castration-induced fat deposition) | GHR methylation rates in the liver of castrated and non-castrated pigs were 93.33% and 0%, respectively | Wang et al., 2017c |
| Duroc and Pietrain pig breeds differing in metabolic characters (muscle metabolism) | More than 2,000 DMCs found in longissimus dorsi muscles | Ponsuksili et al., 2019 |
| Pigs with high or low boar taint (boar taint) | A total of 32 DMCs found in testis | Wang and Kadarmideen, 2019 |
| Hy-Line Brown commercial female chickens before and after reproductive maturation (egg production) | Increased methylation of two CpG sites in ERα; increased H3K27ac and decreased H3K36me3 related to increased ERα mRNA transcript in ovaries | Guo et al., 2020 |
| Betaine supplementation of laying hens (egg production) | Hypomethylation of promoter in GR gene in liver | Omer et al., 2018 |
| Different generations of cashmere goats (cashmere traits) | 753 hypomethylated and 336 hypermethylated 5mC, corresponding to 560 hypomethylated and 214 hypermethylated genes in skin | Dai et al., 2019 |
DMC, differentially methylated cytosine, DMR, differentially methylated region; H3K27ac, acetylation at the 27th lysine residue of histone H3 protein; H3K36me3, tri-methylation at the 36th lysine residue of histone H3 protein.
Disease resistance is influenced by epigenetic factors in farm animals
Disease resistance can be divided into the broad sense and narrow sense. The broad sense refers to disease resistance and disease resilience as well as the tolerance and adaptability to adverse environments (Knap and Doeschl-Wilson, 2020). In a narrow sense, disease resistance mainly refers to the ability of the animal to reduce the proliferation rate of pathogens by inhibiting their infection mechanisms, which is not only related to the host’s resistance to a particular pathogen but also closely related to the host–pathogen–environment interactions (Xu et al., 2020).
Most of the disease-resistant traits, such as general or specific antibody levels, are lowly heritable traits. In addition to genetic factors, environment (including pathogens, management practices, nutrients, etc.) and epigenetic modifications are all influencing factors of animal health- and disease-resistant traits (Figure 3). In recent years, more and more attention has been paid to the regulatory mechanisms and potential function of host epigenetic modifications on animal health and disease resistance traits.
Tackling mastitis resistance of dairy livestock as an example.
Dairy is among the most dynamic and fastest-growing industries in terms of both animal husbandry and the food sector. Milk quality is closely related to public health since milk and dairy products have become an indispensable source of nutrition all over the world. Milk quality and production are easily decreased by many factors, including mastitis (an inflammation of the mammary gland).
Mastitis is one of the most important diseases that affect the sustainable development of the dairy industry. The main cause of mastitis is pathogenic microorganisms that enter the mammary tissue through the nipple duct or skin trauma. According to clinical manifestations, mastitis can be divided into clinical and subclinical mastitis. Mastitis of dairy cows not only affects milk yield and quality, but also abortion and culling rates of cows, leading to huge economic losses to the modern dairy industry.
Like most complex diseases, bovine mastitis resistance is influenced by many factors, including host genetic background, pathogens, epigenetic factors, and the environment. In the prevention and treatment of cow mastitis, antibiotic treatment is prone to induce drug resistance, thus, efforts are geared towards eradicating mastitis through disease resistance breeding. The low heritability of mastitis resistance (<0.1) makes breeding for this trait difficult; complimenting genetic with epigenetic information could provide an avenue for further improvement of mastitis resistance (Figure 2).
In recent times, ample evidence indicates that epigenetic modification plays an important role in the occurrence and development of inflammation (Lee et al., 2006). Studies of epigenetic regulation on inflammatory diseases have brought to light new ideas and research methods for the investigation of regulatory mechanisms in mastitis resistance. In response to inflammation, the host produces corresponding cytokines or immune-related genes to modulate inflammation progression. DNA methylation plays a key role in the regulation of gene expression. In dairy cattle, pilot studies observed that DNA methylation changes are related to the occurrence and development of acute and clinical mastitis (Vanselow et al., 2006; Wang et al., 2013; Wang and Ibeagha-Awemu, 2021). The promoter hypermethylation of the αs1-casein gene and abrupt shutdown of the expression of the gene has been shown in mammary gland tissue of dairy cows with acute mastitis induced by E. coli (Escherichia coli; Vanselow et al., 2006). Moreover, hypermethylation of the promoter region of CD4 gene in peripheral blood cells may inhibit the expression of the gene in clinical mastitic Holstein cows (Wang et al., 2013).
In recent years, more studies have been devoted to the exploration of transcriptome and epigenetic regulatory mechanisms of S. aureus (Staphylococcus aureus) mastitis in dairy cattle, aiming to find effective biomarkers for mastitis resistance selection and molecular diagnosis. S. aureus is one of the main causes of subclinical mastitis. Compared with other bacterial infections, the symptoms of S. aureus mastitis are mild, even asymptomatic, persist for long periods, and show troublesome drug resistance. Moreover, S. aureus can spread in the cattle barn during milking, which makes the prevention and control of S. aureus mastitis more difficult. Song et al. (2016) observed genome-wide DNA methylation differences in peripheral blood lymphocytes between Holstein cows with S. aureus mastitis and healthy cow. The authors further that, the DNA methylation levels of NRG1 [neuregulin 1], MST1 [macrophage stimulating 1], and NAT9 [N-acetyltransferase 9] genes were strongly correlated with the progression of S. aureus subclinical mastitis, making them potential powerful epigenetic markers that can be used to improve the resistance of cows to S. aureus mastitis. Genome-wide DNA methylation patterns in the form of CCGG (CpG)/CCWGG (where W = A or T) (non CpG) were mapped and analyzed in mammary gland quarters of Holstein cows experimentally induced with S. aureus bacteria (Wang et al., 2020). The results further proved that DNA methylation is one of the mechanisms regulating gene expression in bovine mammary gland processes during S. aureus mastitis. Moreover, a series of immune-related differentially methylated genes in “Th17 cell differentiation” and “B-cell receptor signaling” pathways may be candidate genes for S. aureus mastitis (Wang et al., 2020). Further examples of DNA methylation changes related to mastitis are shown in Table 3. DNA methylation changes due to various pathogens causing various livestock diseases, such as Mycobacterium avium subsp. paratuberculosis, Mycobacterium bovis, Porcine epidemic diarrhea virus, and so on, are summarized in Table 3.
Table 3.
Examples of impact of epigenetic changes due to various pathogens on livestock health
| Disease phenotype | Epigenetic changes | References |
|---|---|---|
| Holstein-Friesian cattle infected by Mycobacterium bovis | Genome-wide DNA methylation profile revealed 760 DMRs in CD4+ T cells | Doherty et al., 2016 |
| Holstein cows with subclinical Mycobacterium avium subsp. paratuberculosis infection or Johne’s disease | 2,000 and 6,394 DMCs reported in ileum and ileum lymph node tissues, respectively | Ibeagha-Awemu et al., 2020a; Ibeagha-Awemu et al., 2020b |
| Holstein cows with E. coli or S. aureus mastitis | Many DMRs identified in mammary gland tissues or peripheral blood from Holstein cows | Song et al., 2016; Ju et al., 2020; Wang et al., 2020 |
| Cows with subclinical S. aureus mastitis | Elevated levels of H3K27me3 levels in silent genes in lymphocytes from subclinical S. aureus mastitic cows | He et al., 2016 |
| LPS challenge of endometrial cells from cows | Decreased methylation of specific CpG sites in IL-6 and increased expression levels of IL-6, IL-8, and DNMT genes | Wang et al., 2018 |
| E. coli challenge of mammary epithelial cells from sows | A total of 561 and 898 DMCs identified at 3 h and 24 h after E. coli challenge | Sajjanar et al., 2019 |
| Porcine epidemic diarrhea virus in Large White piglets | Higher H3K4me3 enrichment and expression levels of some antiviral genes in infected jejunum tissue | Wang et al., 2019a |
| Domestic chickens infected with Salmonella enterica | A total of 879 DMRs including 135 DMRs found in the promoter regions of genes in the blood | Wang et al., 2017a |
| Marek’s disease resistant and susceptible White Leghorn | Altered H3K27me3 marks in the bursa of Fabricius were associated with immune-related pathways (e.g., MAP kinase signaling, focal adhesion, and neuroactive ligand–receptor interaction) | Mitra et al., 2015 |
| NDV infection under heat stress condition of Leghorns and Fayoumis | Higher differences in histone modification (H3K27ac and H3K4me1) levels in bursa from Leghorns than Fayoumis, associated genes enriched in biological processes gene ontology terms related to cell cycle and receptor signaling of lymphocytes | Chanthavixay et al., 2020 |
DMR, differentially methylated region; DMC, differentially methylated cytosine; H3K4me3, tri-methylation at the 4th lysine residue of histone H3 protein; H3K27me3, tri-methylation at the 27th lysine residue of histone H3 protein; E. coli, Escherichia coli; S. aureus, Staphylococcus aureus; LPS, lipopolysaccharide; NDV, Newcastle disease virus; H3K27ac, acetylation at the 27th lysine residue of histone H3 protein; H3K4me1, the mono-methylation at the 4th lysine residue of the histone H3 protein.
Apart from DNA methylation, miRNA is another important epigenetic modification that posttranscrioptionally regulate the expression of genes. By interrogating genome-wide expression of mRNAs (messenger RNA) and miRNAs in bovine mammary gland cells at 24 h after intra-mammary infection with high or low concentrations of S. aureus, Fang et al. (2016) found that, compared to the uninfected quarters, significant upregulation of bta-mir-223 and bta-mir-21-3p were observed in the quarters of cows infected with a high concentration of S. aureus. Network analysis suggested that the pivotal role of these miRNAs in protecting host against S. aureus infection is probably through inhibition of CXCL14 [C-X-C motif chemokine ligand 14] and KIT [KIT proto-oncogene, receptor tyrosine kinase] genes (Fang et al., 2016). Moreover, transcriptome sequencing also found four differentially expressed miRNAs (bta-miR-2339, miR-499, miR-23a, and miR-99b) in S. aureus challenged bovine mammary gland epithelial cells (MAC-T cells; Jin et al., 2014). Important miRNAs associated with mastitis and other livestock diseases have been presented in detail in Do et al. (2021).
These findings demonstrate how the multiple regulations of some key DNA methylation markers, hub miRNAs, and vital genes might influence multiple important pathways leading to phenotypic changes, and also confirmed that the response of the mammary gland and other tissues to pathogenic infection is regulated by a complex network of epigenetic marker-gene-pathway interplay.
How Can Epigenetic Processes Be Exploited to Improve Livestock Health and Productivity?
It is important to understand how a process is affected by a factor, but most importantly is how to use that factor to positively influence that process. It is common knowledge as discussed above that epigenetic processes have varying effects on livestock production and health. Of importance for the future of livestock productivity is how to harness these varying effects for improved livestock productivity and health management.
As discussed in the sections above, epigenetic factors respond to different environmental signals (Figure 3) and interact with the underlying genetic composition during an individual’s lifetime and therefore represent the evolution of individual phenotype variations at specific time points or growth stages. If carefully considered, the relationship between livestock traits and epigenetic markers can lead to the development of epigenetic biomarkers for livestock improved management, as well as further enhance genetic gain in the breeder’s equation, resulting in livestock improvement. For example, the effects of environmental factors such as farm environment, feed quality and quantity, and so on, captured through epigenetic association studies can be developed and included in livestock management approaches.
Potential application of epigenetic data in livestock management
Some potential applications of epigenetic biomarkers in livestock management were stated recently (Wang and Ibeagha-Awemu, 2021):
(1) Epigenetic biomarkers can be used to manage health: Epigenetic factors shown to associate significantly with livestock diseases (see examples in Table 3) can be developed for use in diagnostic, prognostic, predictive, or therapy monitoring of livestock diseases. For example, developed epigenetics biomarkers could play important roles in the management of chronic and silent livestock diseases with no obvious clinical symptoms, such as porcine muscular degenerative disease, metabolic disorders, chronic mastitis, subacute ruminal acidosis, and paratuberculosis. For human health management, DNMT1 (DNA methyltransferase 1) inhibitors such as 5-azacytidine and 5-aza-2′-deoxycytidine have been approved for use as epigenetic drugs by the United States Food and Drug Administration (FDA), and they have demonstrated high efficiency in the treatment of hematological malignancies (Montalvo-Casimiro et al., 2020). For disease diagnosis, a FDA-approved cancer DNA methylation assay (test) based on SEPT9 (septin 9) DNA methylation alteration demonstrates sensitivity of 71.1% to 95.6% and specificity of 81.5% to 99% to diagnose colorectal cancer (Tanić and Beck, 2017).
(2) Epigenetic biomarkers can be used to manage livestock reproduction, growth, and production: Epigenetic biomarkers associated with livestock reproduction, productivity, and health help to provide inside into their interaction with other organismal processes (e.g., biological, molecular, cellular, and immune mechanisms) to shape phenotype outcome, and can be exploited for livestock management. As shown in Tables 1 and 2, specific epigenetic factors are associated with reproduction, growth and development phenotypes, and product phenotypes in response to paternal environment, nutrition, environmental conditions, disease pathogens, and so on. For instance, epigenetic biomarkers in sperm can support sperm quality analysis and selection of breeding males. Moreover, placenta and embryo biopsies as well as newborn blood can find application as predictive biomarkers for future phenotypes of interest in adult life. Knowledge of changes of epigenetic signature due to nutrition, environmental conditions, and so on, during life, may serve to predict responses to all kinds of environmental stressors and implementation of countermeasures.
(3) Epigenetic biomarkers can be used for livestock trait improvement: Since it is common knowledge that genetic data alone do not explain all the phenotypic variance in livestock traits, the black box represented by epigenetics and other factors offers avenues to further improve the genetic gain in Figure 1. Ample evidence of transgenerational epigenetic inheritance has been recorded for livestock traits (Miska and Ferguson-Smith, 2016; Triantaphyllopoulos et al., 2016; Perez and Lehner, 2019). Using somatic cell score (SCS) as a proxy for mammary gland health, Swartz et al. (2021) studied the lactation records of 15,992 daughters from 4,366 herds (first lactation) or 3,570 daughters from 1,554 herds (second lactation), and found that daughters born to first lactation cows with elevated SCS demonstrated decline in fat yield. The authors opined that a few underlying mechanisms including predisposition to mastitis pathogens and changes in the epigenome probably played a role in controlling milk fat synthesis in the daughters. This example demonstrates that maternal stress due to disease pathogens resulting in alterations in the epigenome was partly responsible for the observation in the daughters and therefore exemplifies an intergenerational effect on fat yield.
Certain aspects must be taken into account when considering epigenetic information for livestock improvement (Ibeagha-Awemu and Khatib, 2017): (1) the nature of the epigenetic variation-intragenerational (transient) or transgenerational/intergenerational (heritable); (2) dependence or independence of the epigenetic effect on DNA variant effect; and (3) the causal or consequential effect of the epigenetic effect. Therefore, the nature and source of the epigenetic variation must be clearly defined and applied appropriately. While the transient epigenetic effects can be exploited for routine management, transgenerational effects can be factored into the calculation of GEBV (genomic estimated breeding values). This is in line with the proposition that genetic effects and nongenetic inheritance effects should be dissevered and built in the assessment of trait heritability (Danchin et al., 2011).
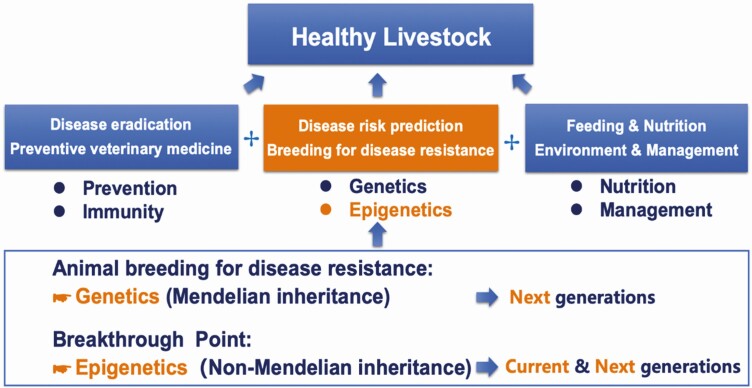
Practical directions for incorporating epigenetic data for improving disease resistance in farm animals
The improvement of animal health can be carried out from the combination of three main aspects: (1) disease elimination and vaccination from the perspective of treatment, prevention, and immunization; (2) optimization of nutritional needs and improvement of environmental sanitation from the perspective of nutrition and management; (3) animal breeding for disease resistance from the perspective of incorporating genetic and epigenetic data in breeding programs for disease resistance (Figure 4).
Figure 4.
Strategies for incorporating genetic and epigenetic data for breeding for disease resistance in livestock.
Among these strategies, the influence of breeding for disease resistance is fundamental. Breeding for disease resistance includes two main points. One point is from the perspective of genetics, which mainly considers the genetic variation in Mendel’s genetic law. That is to say, the health of the population can be steadily improved if individuals with disease resistance from a given generation are mated together to form the next generation. At the same time, the epigenetic regulatory mechanisms due to non-Mendelian inheritance should be effectively exploited for the improvement of disease resistance. Because of the plasticity as well as intergenerational and transgenerational inheritance of epigenetics (Perez and Lehner, 2019), the health status of both present and next generations can be improved by using the characters and mechanisms of epigenetics. Therefore, considering the genetic mechanisms of Mendelian inheritance and the epigenetic mechanisms of non-Mendelian inheritance, we can promote the enhancement of animal health in a timely and long-term way from the present generation and the next generations. In these two ways, the disease resistance of high-yielding farm animals can be well maintained and improved.
Conclusion
In conclusion, livestock are exposed to many factors during a production circle and these factors interact with the epigenome and underlying genetic constitution to determine phenotypic expression. Epigenetic factors are involved in the modulation of livestock reproduction, growth, productivity, and health. The altered epigenetic factors associated to various livestock traits are additional relevant layers of information that can be exploited for livestock management and improvement.
Acknowledgments
Funding for this study was provided by Agriculture and Agri-Food Canada (#J002223).
About the Authors

Eveline M. Ibeagha-Awemu is a senior research scientist with Agriculture and Agri-Food Canada. Her educational journey includes a master’s degree in Animal Genetics and Breeding (University of Nigeria, Nsukka), a doctorate degree in Molecular Genetics (Justus Liebig University, Giessen, Germany), and postdoctoral studies in bovine genomics (McGill University, Canada). She has established national and international reputation for her research in animal genomics and epigenomics. Ibeagha-Awemu’s research focuses on applying OMICs and emerging technologies to unravel the molecular mechanisms of lactation, detect genetic and epigenetic markers of production and health traits, uncover the contribution of epigenetic factors to phenotypic variation, unravel the molecular mechanisms underlying bovine diseases, and develop next-generation biomarkers and therapeutics for bovine health management. Internationally, she is cooperating with scientists in Africa and elsewhere to establish an African Animal Breeding Network to facilitate sustainable livestock genetic improvement in Africa. She has participated in expert consultancy meetings and panels, expert committees, and professional societies including holding executive position as President of the Canadian Society of Animal Science.

Ying Yu is a professor and associate chair of the Department of Animal Breeding and Genetics at College of Animal Science and Technology, China Agricultural University. She is a member of the Farm Animal Genotype-Tissue Expression (FarmGTEx) consortium. Ying holds a PhD in Animal Breeding and Genetics from China Agricultural University. She completed a postdoctoral fellowship at University of Maryland, College Park, USA. She has served as the Executive Chairman of 2020 online workshop on Epigenetic Regulations and Animal and Human Health, and secretary-general of the 2018 International Symposium on Animal Health and Inheritance of Disease Resistance. Her research currently focuses on genetic and epigenetic regulatory mechanisms of animal health and sperm quality; risk assessment of common diseases in farm animals; mechanisms of inter-/transgenerational epigenetic inheritance and its application in breeding animals for disease resistance.
Conflict of interest statement. The authors declare that there is no conflict of interest.
Literature Cited
- Abobaker, H., Hu Y., Omer N.A., Hou Z., Idriss A.A., and Zhao R.. . 2019. Maternal betaine suppresses adrenal expression of cholesterol trafficking genes and decreases plasma corticosterone concentration in offspring pullets. J. Anim. Sci. Biotechnol. 10:87. doi: 10.1186/s40104-019-0396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alharthi, A.S., Coleman D.N., Liang Y., Batistel F., Elolimy A.A., Yambao R.C., Abdel-Hamied E., Pan Y.X., Parys C., Alhidary I.A., . et al. 2019. Hepatic 1-carbon metabolism enzyme activity, intermediate metabolites, and growth in neonatal Holstein dairy calves are altered by maternal supply of methionine during late pregnancy. J. Dairy Sci. 102:10291–10303. doi: 10.3168/jds.2019-16562. [DOI] [PubMed] [Google Scholar]
- Bird, A. 2002. DNA methylation patterns and epigenetic memory. Genes Dev. 16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- Chang, G., Zhang K., Xu T., Jin D., Guo J., Zhuang S., and Shen X.. . 2015. Epigenetic mechanisms contribute to the expression of immune related genes in the livers of dairy cows fed a high concentrate diet. PLoS One. 10:e0123942. doi: 10.1371/journal.pone.0123942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanthavixay, G., Kern C., Wang Y., Saelao P., Lamont S.J., Gallardo R.A., Rincon G., and Zhou H.. . 2020. Integrated transcriptome and histone modification analysis reveals NDV infection under heat stress affects bursa development and proliferation in susceptible chicken line. Front. Genet. 11:1176. doi: 10.3389/fgene.2020.567812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Z., Chu S., Xu X., Jiang J., Wang W., Shen H., Li M., Zhang H., Mao Y., and Yang Z.. . 2019. Analysis of longissimus muscle quality characteristics and associations with DNA methylation status in cattle. Genes Genomics. 41:1147–1163. doi: 10.1007/s13258-019-00844-4. [DOI] [PubMed] [Google Scholar]
- Corbett, R.J., Luttman A.M., Wurtz K.E., Siegford J.M., Raney N.E., Ford L.M., and Ernst C.W.. . 2021. Weaning induces stress-dependent DNA methylation and transcriptional changes in piglet PBMCs. Front. Genet. 12:633564. doi: 10.3389/fgene.2021.633564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai, B., Zhang M., Yuan J.-L., Ren L.-Q., Han X.-Y., and Liu D.-J.. . 2019. Integrative analysis of methylation and transcriptional profiles to reveal the genetic stability of Cashmere traits in the Tβ4 overexpression of Cashmere goats. Animals. 9(12):1002. doi: 10.3390/ani9121002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danchin, É., Charmantier A., Champagne F.A., Mesoudi A., Pujol B., and Blanchet S.. . 2011. Beyond DNA: integrating inclusive inheritance into an extended theory of evolution. Nat. Rev. Genet. 12:475–486. doi: 10.1038/nrg3028. [DOI] [PubMed] [Google Scholar]
- Dechow, C.D., and Liu W.S.. . 2018. DNA methylation patterns in peripheral blood mononuclear cells from Holstein cattle with variable milk yield. BMC Genomics. 19:744. doi: 10.1186/s12864-018-5124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Corvo, M., Lazzari B., Capra E., Zavarez L., Milanesi M., Utsunomiya Y.T., Utsunomiya A.T.H., Stella A., de Paula Nogueira G., Garcia J.F., . et al. 2021. Methylome patterns of cattle adaptation to heat stress. Front. Genet. 12:633132. doi: 10.3389/fgene.2021.633132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do, D.N., Dudemaine P.-L., Mathur M., Suravajhala P., Zhao X., and Ibeagha-Awemu E.M.. . 2021. MiRNA regulatory functions in farm animal diseases, and biomarker potentials for effective therapies. Int. J. Mol. Sci. 22(6):3080. doi: 10.3390/ijms22063080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do, D.N., and Ibeagha-Awemu E.M.. . 2017. Non-coding RNA roles in ruminant mammary gland development and lactation. In: I. Gigli, editor. Current topics in lactation. Rijeka: InTech; p. Ch. 05. [Google Scholar]
- Do, D.N., Li R., Dudemaine P.L., and Ibeagha-Awemu E.M.. . 2017. MicroRNA roles in signalling during lactation: an insight from differential expression, time course and pathway analyses of deep sequence data. Sci. Rep. 7:44605. doi: 10.1038/srep44605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty, R., Whiston R., Cormican P., Finlay E.K., Couldrey C., Brady C., O’Farrelly C., and Meade K.G.. . 2016. The CD4+ T cell methylome contributes to a distinct CD4+ T cell transcriptional signature in Mycobacterium bovis-infected cattle. Sci. Rep. 6:31014. doi: 10.1038/srep31014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, Y., Liang Y., Deng K., Zhang Z., Zhang G., Zhang Y., and Wang F.. . 2020. Analysis of DNA methylation profiles during sheep skeletal muscle development using whole-genome bisulfite sequencing. BMC Genomics. 21:327. doi: 10.1186/s12864-020-6751-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, L., Hou Y., An J., Li B., Song M., Wang X., Sørensen P., Dong Y., Liu C., Wang Y., . et al. 2016. Genome-wide transcriptional and post-transcriptional regulation of innate immune and defense responses of bovine mammary gland to Staphylococcus aureus. Front. Cell. Infect. Microbiol. 6:193. doi: 10.3389/fcimb.2016.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, X., Zhao Z., Yu H., Li G., Jiang P., Yang Y., Yang R., and Yu X.. . 2017. Comparative genome-wide methylation analysis of longissimus dorsi muscles between Japanese black (Wagyu) and Chinese Red Steppes cattle. PLoS One. 12:e0182492. doi: 10.1371/journal.pone.0182492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, T., Luo F., and Lin Q.. . 2020. You are affected by what your parents eat: diet, epigenetics, transgeneration and intergeneration. Trends Food Sci. Technol. 100:248–261. doi: 10.1016/j.jpgs.2020.04.021. [DOI] [Google Scholar]
- Han, W., Yang F., Wu Z., Guo F., Zhang J., Hai E., Shang F., Su R., Wang R., Wang Z., . et al. 2020. Inner Mongolian Cashmere Goat secondary follicle development regulation research based on mRNA-miRNA co-analysis. Sci. Rep. 10:4519. doi: 10.1038/s41598-020-60351-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He, Y., Song M., Zhang Y., Li X., Song J., Zhang Y., and Yu Y.. . 2016. Whole genome regulation analysis of histone H3 lysin 27 trimethylation in subclinical mastitis cows infected by Staphylococcus aureus. BMC Genomics. 17:565. doi: 10.1186/s12864-016-2947-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, Y., Sun Q., Zong Y., Liu J., Idriss A.A., Omer N.A., and Zhao R.. . 2017. Prenatal betaine exposure alleviates corticosterone-induced inhibition of CYP27A1 expression in the liver of juvenile chickens associated with its promoter DNA methylation. Gen. Comp. Endocrinol. 246:241–248. doi: 10.1016/j.ygcen.2016.12.014. [DOI] [PubMed] [Google Scholar]
- Ibeagha-Awemu, E.M., and Khatib H.. . 2017. Epigenetics of livestock breeding. In: T. O. Tollefsbol, editor. Handbook of epigenetics: the new molecular and medical genetics. London, United Kingdom: Academic Press; p. 441–463. doi: 10.1016/B978-0-12-805388-1.00029-8. [DOI] [Google Scholar]
- Ibeagha-Awemu, E.M., and Zhao X.. . 2015. Epigenetic marks: regulators of livestock phenotypes and conceivable sources of missing variation in livestock improvement programs. Front. Genet. 6:302. doi: 10.3389/fgene.2015.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibeagha-Awemu, E.M., Bhattarai S., Dedemaine P.-L., Wang M., Mckay S.D., Zhao X., and Bissonnette N.. . 2020a. 63 DNA methylome wide profile associates differentially methylated loci and regions with cow’s ileal lymph node response to Mycobacterium avium subsp. paratuberculosis. J. Anim. Sci. 98:39–40. doi: 10.1093/jas/skaa278.071. [DOI] [Google Scholar]
- Ibeagha-Awemu, E.M., Bhattarai S., Dedemaine P.-L., Wang M., Mckay S.D., Zhao X., and Bissonnette N.. . 2020b. PSVIII-15 Genome wide DNA methylation analysis reveals role of DNA methylation in cow’s ileal response to Mycobacterium avium subsp. paratuberculosis. J. Anim. Sci. 98:260–261. doi:10.1093/jas/ skaa278.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ideraabdullah, F.Y., and Zeisel S.H.. . 2018. Dietary modulation of the epigenome. Physiol. Rev. 98:667–695. doi: 10.1152/physrev.00010.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, W., Ibeagha-Awemu E.M., Liang G., Beaudoin F., Zhao X., and Guan L.L.. . 2014. Transcriptome microRNA profiling of bovine mammary epithelial cells challenged with Escherichia coli or Staphylococcus aureus bacteria reveals pathogen directed microRNA expression profiles. BMC Genomics. 15:181. doi: 10.1186/1471-2164-15-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, C., Zhuo Y., Wang J., Zhao Y., Xuan Y., Mou D., Liu H., Zhou P., Fang Z., Che L., . et al. 2018. Methyl donors dietary supplementation to gestating sows diet improves the growth rate of offspring and is associating with changes in expression and DNA methylation of insulin-like growth factor-1 gene. J. Anim. Physiol. Anim. Nutr. 102:1340–1350. doi: 10.1111/jpn.12933. [DOI] [PubMed] [Google Scholar]
- Ju, Z., Jiang Q., Wang J., Wang X., Yang C., Sun Y., Zhang Y., Wang C., Gao Y., Wei X., . et al. 2020. Genome-wide methylation and transcriptome of blood neutrophils reveal the roles of DNA methylation in affecting transcription of protein-coding genes and miRNAs in E. coli-infected mastitis cows. BMC Genomics. 21:102. doi: 10.1186/s12864-020-6526-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knap, P.W., and Doeschl-Wilson A.. . 2020. Why breed disease-resilient livestock, and how? Genet. Sel. Evol. 52:60. doi: 10.1186/s12711-020-00580-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides, T. 2007. Chromatin modifications and their function. Cell. 128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Langevin, S.M., and Kelsey K.T.. . 2013. The fate is not always written in the genes: epigenomics in epidemiologic studies. Environ. Mol. Mutagen. 54:533–541. doi: 10.1002/em.21762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, G.R., Kim S.T., Spilianakis C.G., Fields P.E., and Flavell R.A.. . 2006. T helper cell differentiation: regulation by cis elements and epigenetics. Immunity. 24:369–379. doi: 10.1016/j.immuni.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Li, Y., Carrillo J.A., Ding Y., He Y., Zhao C., Liu J., Zan L., and Song J.. . 2019. DNA methylation, microRNA expression profiles and their relationships with transcriptome in grass-fed and grain-fed angus cattle rumen tissue. PLoS One. 14:e0214559. doi: 10.1371/journal.pone.0214559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlejohn, B.P., Price D.M., Neuendorff D.A., Carroll J.A., Vann R.C., Riggs P.K., Riley D.G., Long C.R., T.H.Welsh, Jr, and Randel R.D.. . 2018. Prenatal transportation stress alters genome-wide DNA methylation in suckling Brahman bull calves. J. Anim. Sci. 96:5075–5099. doi: 10.1093/jas/sky350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv, Z., Fan, H., Song, B., Li, G., Liu, D. and Guo Y.. . 2019. Supplementing genistein for breeder hens alters the fatty acid metabolism and growth performance of offsprings by epigenetic modification. Oxid. Med. Cell. Longev. 2019:e9214209. doi: 10.1155/2019/9214209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miska, E.A., and Ferguson-Smith A.C.. . 2016. Transgenerational inheritance: models and mechanisms of non-DNA sequence-based inheritance. Science. 354:59–63. doi: 10.1126/science.aaf4945. [DOI] [PubMed] [Google Scholar]
- Mitra, A., Luo J., He Y., Gu Y., Zhang H., Zhao K., Cui K., and Song J.. . 2015. Histone modifications induced by MDV infection at early cytolytic and latency phases. BMC Genomics. 16:311. doi: 10.1186/s12864-015-1492-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalvo-Casimiro, M., González-Barrios R., Meraz-Rodriguez M.A., Juárez-González V.T., Arriaga-Canon C., and Herrera L.A.. . 2020. Epidrug repurposing: discovering new faces of old acquaintances in cancer therapy. Front. Oncol. 10:2461. doi: 10.3389/fonc.2020.605386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, K.V., and Mattick J.S.. . 2014. The rise of regulatory RNA. Nat. Rev. Genet. 15:423–437. doi: 10.1038/nrg3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, M., Boutinaud M., Pétridou B., Gabory A., Pannetier M., Chat S., Bouet S., Jouneau L., Jaffrezic F., Laloë D., . et al. 2014. DNA methylation and transcription in a distal region upstream from the bovine AlphaS1 casein gene after once or twice daily milking. PLoS One. 9:e111556. doi: 10.1371/journal.pone.0111556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omer, N.A., Hu Y., Hu Y., Idriss A.A., Abobaker H., Hou Z., Dong H., and Zhao R.. . 2018. Dietary betaine activates hepatic VTGII expression in laying hens associated with hypomethylation of GR gene promoter and enhanced GR expression. J. Anim. Sci. Biotechnol. 9:2. doi: 10.1186/s40104-017-0218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio, J.S., Jacometo C.B., Zhou Z., Luchini D., Cardoso F.C., and Loor J.J.. . 2016. Hepatic global DNA and peroxisome proliferator-activated receptor alpha promoter methylation are altered in peripartal dairy cows fed rumen-protected methionine. J. Dairy Sci. 99:234–244. doi: 10.3168/jds.2015-10157. [DOI] [PubMed] [Google Scholar]
- Paradis, F., Wood K.M., Swanson K.C., Miller S.P., McBride B.W., and Fitzsimmons C.. . 2017. Maternal nutrient restriction in mid-to-late gestation influences fetal mRNA expression in muscle tissues in beef cattle. BMC Genomics. 18:632. doi: 10.1186/s12864-017-4051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez, M.F., and Lehner B.. . 2019. Intergenerational and transgenerational epigenetic inheritance in animals. Nat. Cell Biol. 21:143–151. doi: 10.1038/s41556-018-0242-9. [DOI] [PubMed] [Google Scholar]
- Ponsuksili, S., Trakooljul N., Basavaraj S., Hadlich F., Murani E., and Wimmers K.. . 2019. Epigenome-wide skeletal muscle DNA methylation profiles at the background of distinct metabolic types and ryanodine receptor variation in pigs. BMC Genomics. 20:492. doi: 10.1186/s12864-019-5880-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang, W., Guo H., Li Y., Shi J., Yin X., and Qu J.. . 2018. Methylation analysis of CMTM3 and DUSP1 gene promoters in high-quality brush hair in the Yangtze River delta white goat. Gene. 668:166–173. doi: 10.1016/j.gene.2018.05.031. [DOI] [PubMed] [Google Scholar]
- Sajjanar, B., Trakooljul N., Wimmers K., and Ponsuksili S.. . 2019. DNA methylation analysis of porcine mammary epithelial cells reveals differentially methylated loci associated with immune response against Escherichia coli challenge. BMC Genomics. 20:623. doi: 10.1186/s12864-019-5976-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, M., He Y., Zhou H., Zhang Y., Li X., and Yu Y.. . 2016. Combined analysis of DNA methylome and transcriptome reveal novel candidate genes with susceptibility to bovine Staphylococcus aureus subclinical mastitis. Sci. Rep. 6:29390. doi: 10.1038/srep29390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz, T.H., Bradford B.J., and Clay J.S.. . 2021. Intergenerational cycle of disease: maternal mastitis is associated with poorer daughter performance in dairy cattle. J Dairy Sci. 104:4537–4548. doi: 10.3168/jds.2020-19249. [DOI] [PubMed] [Google Scholar]
- Tanić, M., and Beck S.. . 2017. Epigenome-wide association studies for cancer biomarker discovery in circulating cell-free DNA: technical advances and challenges. Curr. Opin. Genet. Dev. 42:48–55. doi: 10.1016/j.gde.2017.01.017. [DOI] [PubMed] [Google Scholar]
- Tian, P., Luo Y., Li X., Tian J., Tao S., Hua C., Geng Y., Ni Y., and Zhao R.. . 2017. Negative effects of long-term feeding of high-grain diets to lactating goats on milk fat production and composition by regulating gene expression and DNA methylation in the mammary gland. J. Anim. Sci. Biotechnol. 8:74. doi: 10.1186/s40104-017-0204-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triantaphyllopoulos, K.A., Ikonomopoulos I., and Bannister A.J.. . 2016. Epigenetics and inheritance of phenotype variation in livestock. Epigenetics Chromatin. 9:31. doi: 10.1186/s13072-016-0081-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Doormaal, B.V. 2019. 10 Years of Genomic Selection: What’s Next? Lactanet’s Information Articles. Lactanet, Canada. Available from https://www.cdn.ca/document.php?id=530
- Vanselow, J., Yang W., Herrmann J., Zerbe H., Schuberth H.J., Petzl W., Tomek W., and Seyfert H.M.. . 2006. DNA-remethylation around a STAT5-binding enhancer in the alphaS1-casein promoter is associated with abrupt shutdown of alphaS1-casein synthesis during acute mastitis. J. Mol. Endocrinol. 37:463–477. doi: 10.1677/jme.1.02131. [DOI] [PubMed] [Google Scholar]
- Wang, M., and Ibeagha-Awemu E.M.. . 2021. Impacts of epigenetic processes on the health and productivity of livestock. Front. Genet. 11(1812):613636. doi: 10.3389/fgene.2020.613636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X., and Kadarmideen H.. . 2019. Genome-wide DNA methylation analysis using next-generation sequencing to reveal candidate genes responsible for boar taint in pigs. Anim. Genet. 50:644–659. doi: 10.1111/age.12842. [DOI] [PubMed] [Google Scholar]
- Wang, J., Chen J., Zhang J., Gao B., Bai X., Lan Y., Lin P., Guo H., Gao Y., and Xing B.. . 2017C. Castration induced changes in the expression profiles and promoter methylation of the GHR gene in Huainan male pigs. Anim. Sci. J. 88:1113–1119. doi: 10.1111/asj.12739. [DOI] [PubMed] [Google Scholar]
- Wang, M., Liang Y., Ibeagha-Awemu E.M., Li M., Zhang H., Chen Z., Sun Y., Karrow N.A., Yang Z., and Mao Y.. . 2020. Genome-wide DNA methylation analysis of mammary gland tissues from Chinese Holstein cows with Staphylococcus aureus induced mastitis. Front. Genet. 11:1295. doi: 10.3389/fgene.2020.550515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y., Luo M., Jiang M., Lin Y., and Zhu J.. . 2017b. Comparative analysis of tissue expression and methylation reveals the crucial hypoxia genes in hypoxia-resistant animals. Can. J Anim Sci. 98(1):204–212. doi: 10.1139/cjas-2017-0021. [DOI] [Google Scholar]
- Wang, H., Shi H., Luo J., Yi Y., Yao D., Zhang X., Ma G., and Loor J.J.. . 2017a. MiR-145 regulates lipogenesis in goat mammary cells via targeting INSIG1 and epigenetic regulation of lipid-related genes. J. Cell. Physiol. 232:1030–1040. doi: 10.1002/jcp.25499. [DOI] [PubMed] [Google Scholar]
- Wang, L., Sun H., Guan L., and Liu J.. . 2019b. Relationship of blood DNA methylation rate and milk performance in dairy cows. J. Dairy Sci. 102:5208–5211. doi: 10.3168/jds.2018-15869. [DOI] [PubMed] [Google Scholar]
- Wang, J., Yan X., Nesengani L.T., Ding H., Yang L., and Lu W.. . 2018. LPS-induces IL-6 and IL-8 gene expression in bovine endometrial cells “through DNA methylation”. Gene. 677:266–272. doi: 10.1016/j.gene.2018.07.074. [DOI] [PubMed] [Google Scholar]
- Wang, H., Yang L., Qu H., Feng H., Wu S., and Bao W.. . 2019a. Global mapping of H3K4 trimethylation (H3K4me3) and transcriptome analysis reveal genes involved in the response to epidemic diarrhea virus infections in pigs. Animals. 9:523. doi: 10.3390/ani9080523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X.S., Zhang Y., He Y.H., Ma P.P., Fan L.J., Wang Y.C., Zhang Y.I., Sun D.X., Zhang S.L., Wang C.D., . et al. 2013. Aberrant promoter methylation of the CD4 gene in peripheral blood cells of mastitic dairy cows. Genet. Mol. Res. 12:6228–6239. doi: 10.4238/2013.December.4.10. [DOI] [PubMed] [Google Scholar]
- Wiggans, G.R., Cole J.B., Hubbard S.M., and Sonstegard T.S.. . 2017. Genomic selection in dairy cattle: the USDA experience. Annu. Rev. Anim. Biosci. 5:309–327. doi: 10.1146/annurev-animal-021815-111422. [DOI] [PubMed] [Google Scholar]
- Xiao, P., Zhong T., Liu Z., Ding Y., Guan W., He X., Pu Y., Jiang L., Ma Y., and Zhao Q.. . 2019. Integrated analysis of methylome and transcriptome changes reveals the underlying regulatory signatures driving curly wool transformation in Chinese Zhongwei goats. Front. Genet. 10:1263. doi: 10.3389/fgene.2019.01263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, F., Fu Y., Sun T.Y., Jiang Z., Miao Z., Shuai M., Gou W., Ling C.W., Yang J., Wang J., . et al. 2020. The interplay between host genetics and the gut microbiome reveals common and distinct microbiome features for complex human diseases. Microbiome. 8:145. doi: 10.1186/s40168-020-00923-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue, J., Schoenrock S.A., Valdar W., Tarantino L.M., and Ideraabdullah F.Y.. . 2016. Maternal vitamin D depletion alters DNA methylation at imprinted loci in multiple generations. Clin. Epigenetics. 8:107. doi: 10.1186/s13148-016-0276-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, X.-X., Liu Y.-H., Liu X.-M., Wang P.-C., Liu S., Miao J.-K., Du Z.Q., and Yang C.X.. . 2018. Ascorbic acid induces global epigenetic reprogramming to promote meiotic maturation and developmental competence of porcine oocytes. Sci. Rep. 8:6132. doi: 10.1038/s41598-018-24395-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, B., Ban D., Gou X., Zhang Y., Yang L., Chamba Y., and Zhang H.. . 2019. Genome-wide DNA methylation profiles in Tibetan and Yorkshire pigs under high-altitude hypoxia. J. Anim. Sci. Biotechnol. 10:25. doi: 10.1186/s40104-019-0316-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, M., Li D., Zhai Y., Wang Z., Ma X., Zhang D., Li G., Han R., Jiang R., Li Z., . et al. 2020. The landscape of DNA methylation associated with the transcriptomic network of intramuscular adipocytes generates insight into intramuscular fat deposition in Chicken. Front. Cell Dev. Biol. 8:206. doi: 10.3389/fcell.2020.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, S., Shen L., Xia Y., Yang Q., Li X., Tang G., Jiang Y., Wang J., Li M., and Zhu L.. . 2016. DNA methylation landscape of fat deposits and fatty acid composition in obese and lean pigs. Sci. Rep. 6:35063. doi: 10.1038/srep35063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, M., Yan F.B., Li F., Jiang K.R., Li D.H., Han R.L., Li Z.J., Jiang R.R., Liu X.J., Kang X.T., . et al. 2017a. Genome-wide DNA methylation profiles reveal novel candidate genes associated with meat quality at different age stages in hens. Sci. Rep. 7:45564. doi: 10.1038/srep45564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X., Zhang S., Ma L., Jiang E., Xu H., Chen R., Yang Q., Chen H., Li Z., and Lan X.. . 2017b. Reduced representation bisulfite sequencing (RRBS) of dairy goat mammary glands reveals DNA methylation profiles of integrated genome-wide and critical milk-related genes. Oncotarget. 8:115326–115344. doi: 10.18632/oncotarget.23260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, C., Ji G., Carrillo J.A., Li Y., Tian F., Baldwin R.L., Zan L., and Song J.. . 2020. The profiling of DNA methylation and its regulation on divergent tenderness in angus beef cattle. Front. Genet. 11:939. doi: 10.3389/fgene.2020.00939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, Y., Hui T., Yue C., Sun J., Guo D., Guo S., Guo S., Li B., Wang Z., and Bai W.. . 2020. Comprehensive analysis of circRNAs from Cashmere goat skin by next generation RNA sequencing (RNA-seq). Sci. Rep. 10:516. doi: 10.1038/s41598-019-57404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]