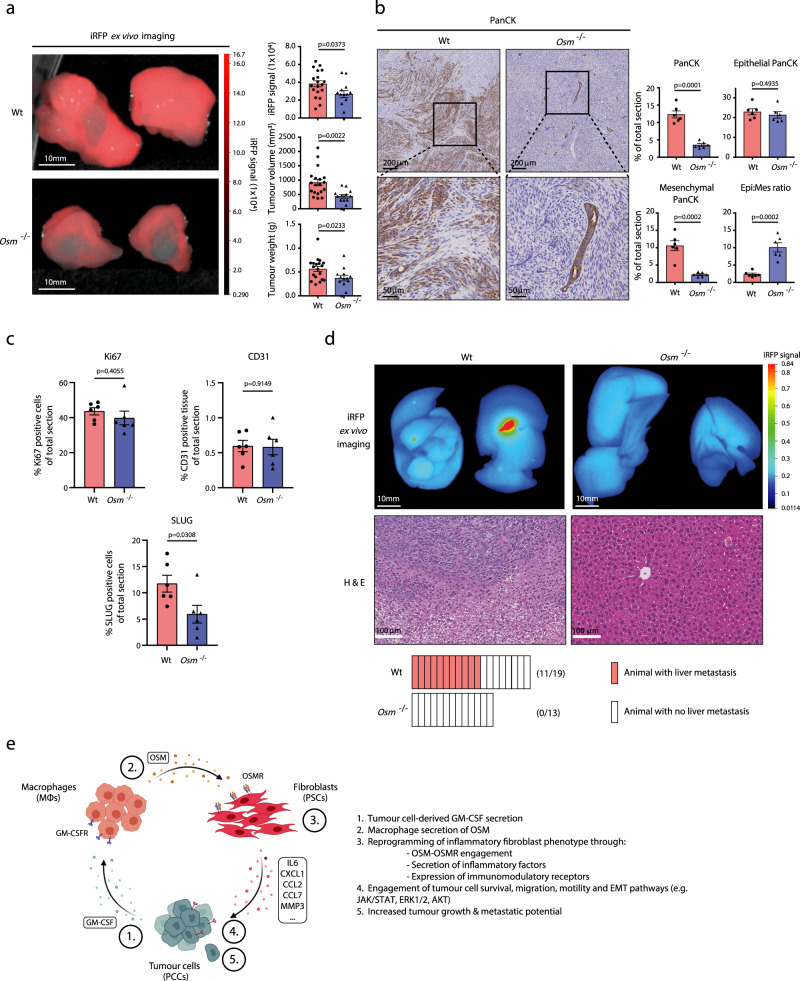
Fig. 7. OSM drives tumour growth and metastasis in vivo.
a Left: Representative iRFP imaging of resected pancreatic tumours from orthotopically transplanted immune-competent wildtype (Wt) (n = 19) and Osm−/− (n = 13) mice. Right: Quantification of iRFP signal intensity, tumour volume and tumour weight. Results displayed as mean ± SEM (Wt, n = 19 and Osm−/− n = 13), two-tailed student t test. b Left: Representative Pan-Cytokeratin (PanCK) staining of wildtype and Osm−/− tumour sections. Right: Quantification of total PanCKpos staining, mesenchymal PanCKpos staining, and epithelial PanCKpos staining following morphology analysis, and the epithelial–mesenchymal PanCKpos ratio in tumour sections from wildtype (n = 6) and Osm−/− (n = 6) mice. A shift of PanCKpos cells from a mixed mesenchymal and epithelial morphology in wildtype tumours to a more epithelial-dominant morphology in Osm−/− animals. Results displayed as mean ± SEM, two-tailed student t test. c Quantification of total Ki67,pos CD31pos, and SLUGpos immunohistochemical staining of tumour sections from wildtype (n = 6) and Osm−/− (n = 6) mice. Results displayed as mean ± SEM, two-tailed student t test. d Upper: Representative iRFP in vivo imaging (top) and haematoxylin and eosin staining (bottom) of resected livers from wildtype and Osm−/− mice. Lower: Quantification of iRFPpos liver metastasis incidents in wildtype (n = 11/19) and Osm−/− (n = 0/13) animals. e Model outlining heterocellular OSM–OSMR signalling between pancreatic cancer cells, fibroblasts and macrophages. Pancreatic cancer cell secretion of GM-CSF induces macrophage secretion of OSM, which reprograms fibroblasts to an inflammatory phenotype, in turn accentuating tumour growth and metastasis. Also see Supplementary Fig. 8. Source data are provided as a Source Data file.