Abstract
Juvenile hormone (JH) plays vital roles in insect reproduction, development, and in many aspects of physiology. JH primarily acts at the gene-regulatory level through interaction with an intracellular receptor (JH receptor [JHR]), a ligand-activated complex of transcription factors consisting of the JH-binding protein methoprene-tolerant (MET) and its partner taiman (TAI). Initial studies indicated significance of post-transcriptional phosphorylation, subunit assembly, and nucleocytoplasmic transport of JHR in JH signaling. However, our knowledge of JHR regulation at the protein level remains rudimentary, partly because of the difficulty of obtaining purified and functional JHR proteins. Here, we present a method for high-yield expression and purification of JHR complexes from two insect species, the beetle T. castaneum and the mosquito Aedes aegypti. Recombinant JHR subunits from each species were coexpressed in an insect cell line using a baculovirus system. MET–TAI complexes were purified through affinity chromatography and anion exchange columns to yield proteins capable of binding both the hormonal ligand (JH III) and DNA bearing cognate JH-response elements. We further examined the beetle JHR complex in greater detail. Biochemical analyses and MS confirmed that T. castaneum JHR was a 1:1 heterodimer consisting of MET and Taiman proteins, stabilized by the JHR agonist ligand methoprene. Phosphoproteomics uncovered multiple phosphorylation sites in the MET protein, some of which were induced by methoprene treatment. Finally, we report a functional bipartite nuclear localization signal, straddled by phosphorylated residues, within the disordered C-terminal region of MET. Our present characterization of the recombinant JHR is an initial step toward understanding JHR structure and function.
Keywords: juvenile hormone, insect, hormone receptor, basic helix–loop–helix/transcription factor, ligand-binding protein, protein purification, protein phosphorylation, nuclear translocation, methoprene, PAS domain
Abbreviations: λPP, lambda protein phosphatase; AaMET, Aedes aegypti methoprene-tolerant; AaTAI, Aedes aegypti taiman; AHR, aryl hydrocarbon receptor; ARNT, aryl hydrocarbon receptor nuclear translocator; bHLH–PAS, basic helix–loop–helix/Per–Arnt–Sim; BSA, bovine serum albumin; EcR, ecdysone receptor; ESI, electrospray ionization; ET, early trypsin; FA, formic acid; FBS, fetal bovine serum; FDR, false discovery rate; GCE, germ cell–expressed; Ha, Helicoverpa armigera; HEK293, human embryonic kidney 293 cell line; HSP, heat shock protein; IMAC, immobilized metal affinity chromatography; JH, juvenile hormone; JHR, juvenile hormone receptor; JHRE, juvenile hormone response element; Kr-h1, Krüppel-homolog 1; MET, methoprene-tolerant; MFBS1, MET–FISC binding site 1; NCBI, National Center for Biotechnology Information; NLS, nuclear localization signal; SEC–MALLS, size-exclusion chromatography in combination with multiangle laser-light scattering; Sf9, Spodoptera frugiperda 9 cell line; SFM, serum-free media; TAI, Taiman; Tc, Tribolium castaneum; TCEP, Tris (2-carboxyethyl) phosphine; TcTAI, Tribolium castaneum taiman; TSAP, thermosensitive alkaline phosphatase; USP, ultraspiracle
Juvenile hormone (JH) and 20-hydroxyecdysone are the two major hormones controlling insect molting and metamorphosis (1, 2). These hormones also regulate a range of developmental and physiological processes, including reproduction, behavior, morphological polyphenism, metabolism, and immunity (3, 4, 5, 6, 7). The nuclear ecdysone receptor (EcR), initially cloned from Drosophila melanogaster (8), has been shown to dimerize with another nuclear receptor ultraspiracle (USP) to form an active 20-hydroxyecdysone receptor (9, 10). Purification of functional recombinant EcR–USP protein complexes from pest insects has revealed the structural mode of the receptor–ligand interaction, explained the order selectivity of EcR-targeting insecticides (11), and aided development of insecticide discovery tools (12).
The nature of the intracellular receptor for JH has proven more problematic with a number of candidates being considered (13). D. melanogaster USP was observed to bind, initially JH III, and subsequently other sesquiterpenoid methyl farnesoids (14), suggesting a mechanism whereby the JH and ecdysone signaling pathways might converge through the EcR–USP complex. However, a JH receptor (JHR) role for USP has proven controversial on several grounds, including the absence of a JH binding pocket in the USP subunits of established ecdysteroid–EcR–USP X-ray structures (15, 16). The observation of a patent ligand-binding pocket in the structure of a hitherto unobserved apo form of an EcR–USP heterodimer (17) may reopen this discussion.
Wilson and Fabian (18) discovered in D. melanogaster a gene, termed Methoprene-tolerant (Met), which confers sensitivity to the insecticide methoprene, a JH mimic. Met and its D. melanogaster paralog germ cell–expressed (gce) encode transcription factors of the basic helix–loop–helix/Per–aryl hydrocarbon receptor nuclear translocator (Arnt)–Sim (bHLH–PAS) family (19, 20). The MET and GCE proteins from D. melanogaster and their orthologs from diverse insect species display properties expected for a JHR (21), including binding of JH III with nanomolar affinities (22, 23, 24, 25). This hormone binding, mediated by a pocket within the PAS-B domain of MET/GCE (23, 24, 25), is highly stereoselective (26). Genetic rescue of nonconditional lethality in D. melanogaster mutants lacking both MET and GCE (27) has revealed that the JH-binding capacity of either protein is required for the flies to complete development (25). As initially demonstrated in larvae of the beetle T. castaneum (28) and later in diverse other insects (29, 30, 31), deficiency of Met function leads to precocious metamorphosis, a phenotype consistent with a deficiency of JH itself. Importantly, MET mediates the effects of JH by directly inducing transcription of genes such as Krüppel-homolog 1 (Kr-h1) whose product(s) repress metamorphosis (29, 32, 33, 34, 35, 36, 37).
To activate transcription, bHLH–PAS factors typically form heterodimers with other members of this protein family (38). Li et al. (39) employed a yeast two-hybrid system to detect in the mosquito, A. aegypti, a bHLH–PAS protein that binds MET in the presence of methoprene. A partner protein has also been identified for MET of T. castaneum (40) and shown to interact with it in a manner depending on JH or methoprene binding to MET (23). Both the mosquito and beetle MET partnering proteins are counterparts of the mammalian steroid receptor coactivator 1, whose first insect homolog was identified in D. melanogaster as the product of a gene taiman (TAI) (41). We will refer to this protein as TAI. MET–TAI complexes, presumed heterodimers, have been shown to associate with JH response elements (JHREs) in the enhancers of Kr-h1, early trypsin (ET), and other JH-responsive genes of A. aegypti (24, 33, 39, 42, 43). RNAi-mediated knockdown in the cockroach Blattella germanica has revealed a role for TAI in suppressing metamorphosis (44). As expected for subunits of a functional JHR, coexpression of MET and TAI has been shown to confer JH responsiveness to otherwise JH nonresponsive mammalian cells (33, 45).
In addition to its direct gene-regulatory action, JH exerts effects initiated at the cell membrane. While investigating protein synthesis in the D. melanogaster male accessory glands, Yamamoto et al. (46) described a pathway involving Ca2+ signaling and a protein kinase C. Synthesis of accessory gland proteins was reduced in Met mutant males (47), suggesting that MET was also involved. Detailed investigations by Liu et al. (48) in A. aegypti have linked JH action through an as yet unknown cell-membrane receptor and Ca2+/second messenger signaling to phosphorylation of MET and TAI, which in turn led to an increased transcriptional activity of the intracellular JHR. Several phosphorylation sites in Aedes aegypti methoprene-tolerant (AaMET) have recently been reported (49). Clearly, understanding of post-translational regulation of the intracellular JHR is of considerable interest. However, purification of a native and functional JHR complex from insect cells has not previously been accomplished.
In this communication, we present high-yield insect cell–based expression and purification of recombinant JHR MET–TAI complexes from T. castaneum and A. aegypti that are active in both hormone and target JHRE DNA binding. We establish the presumed, but hitherto unproven, 1:1 ratio between the receptor protein subunits and identify a conserved signal of nuclear import of MET. Our primary identification of amino acid residues phosphorylated in the active MET–TAI complex paves the way to understanding the role of post-translational regulation in JH signaling.
Results
Expression and purification of JHR proteins from T. castaneum and A. aegypti
The affinity-tagged T. castaneum JH receptor proteins, FLAG-T. castaneum methoprene-tolerant and His6-Tribolium castaneum taiman (TcTAI), were coexpressed in Sf9 insect cells infected with a baculovirus expression construct depicted in Figure 1A. While T. castaneum methoprene-tolerant was expressed in its entirety, TcTAI sequence was limited to include the bHLH and the two tandem PAS domains, that is, regions that are required and sufficient for TcTAI to engage in the JH-stimulated interaction with T. castaneum methoprene-tolerant (23). The tagged T. castaneum JH receptor proteins were purified through successive affinity chromatography steps using immobilized Ni2+ (immobilized metal affinity chromatography [IMAC]) and anti-FLAG antibody, respectively (Fig. 1B). A final anion exchange chromatography step provided further purification and concentration. All the purification steps were performed in the presence of 10 μM methoprene, a JH analog and an agonist ligand of T. castaneum methoprene-tolerant (23). The purified T. castaneum JH receptor is a complex of the FLAG-T. castaneum methoprene-tolerant and His6-TcTAI subunits detected by anti-His6 and anti-FLAG antibodies and exhibiting apparent molecular weights consistent with those predicted from the FLAG-T. castaneum methoprene-tolerant and His6-TcTAI sequences (59.8 and 42.7 kDa, respectively). The yield of the T. castaneum JH receptor protein complex ranged from 1 to 1.5 mg per 10 g of packed cells, that is, 2 to 3 mg per liter of culture.
Figure 1.
Expression and purification of the recombinant JHR proteins of T.castaneum.A, the interacting components of the T. castaneum JH receptor complex, the truncated TcTAI and the full-length T. castaneum methoprene-tolerant proteins, were coexpressed in Sf9 cells from the baculoviral pFastBac Dual construct under the polyhedrin (PH) and p10 promoters, respectively. N-terminal affinity tags and the major functional domains are depicted. B, the T. castaneum JH receptor protein complex was purified from lysates of the baculovirus-infected Sf9 cells in two affinity chromatography steps using immobilized Ni2+ (IMAC) and anti-FLAG antibody, respectively, followed by anion exchange chromatography, all in the presence of 10 μM methoprene. Fractions under “B” containing both T. castaneum methoprene-tolerant and TcTAI were pooled for further work. Top panels show Coomassie-stained SDS-PAGE; below are immunoblots of the same samples developed with the indicated antitag antibodies. F, flow-through; IMAC, immobilized metal affinity chromatography; JHR, juvenile hormone receptor; S, starting lysate; TcTAI, T. castaneum taiman.
A baculovirus construct was also designed for coexpression of tagged A. aegypti JHR proteins His6-AaMET and FLAG-Aedes aegypti taiman (AaTAI) (Fig. S1A) in Sf9 cells. AaMET was N-terminally truncated to remove the first 113 amino acids, whereas AaTAI only contained the bHLH, and both of the PAS domains involved in the JH-dependent interaction with AaMET (39). The AaJHR protein purified as a complex of His6-AaMET and FLAG-AaTAI subunits of apparent molecular weights consistent with those calculated from their sequences in the expression construct (95.1 and 46.8 kDa, respectively) (Fig. S1B). Yields of the purified mosquito protein (0.25–0.5 mg per 10 g of packed cells, 0.5–1 mg per liter of culture) were lower relative to the yields of T. castaneum JH receptor, largely as the result of greater susceptibility to proteolysis (even in the presence of inhibitors) and adsorption to surfaces/reduced solubility.
The purified T. castaneum JH receptor complex is a MET–TAI heterodimer stabilized by methoprene
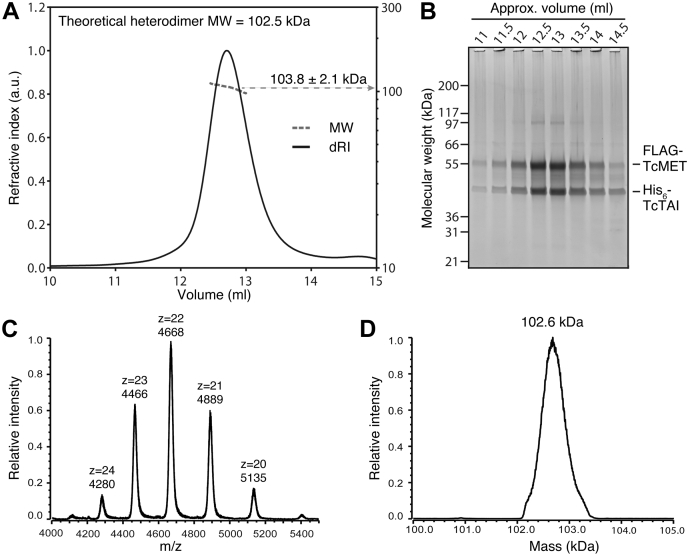
Size-exclusion chromatography in combination with multiangle laser-light scattering (SEC–MALLS) indicated that FLAG-T. castaneum methoprene-tolerant–His6-TcTAI was predominantly a heterodimer in solution with an observed experimental molecular weight of 103.8 ± 2.1 kDa (Fig. 2A; theoretical molecular weights of the FLAG-T. castaneum methoprene-tolerant–His6-TcTAI heterodimer, FLAG-T. castaneum methoprene-tolerant homodimer, and His6-TcTAI homodimer are 102.5, 119.5, and 85.5 kDa, respectively). Post SEC–MALLS, proteins in the fractions including and surrounding the main peak were separated via SDS-PAGE (Fig. 2B), and it was observed that both T. castaneum methoprene-tolerant and TcTAI are present in approximately equal stoichiometric quantities across the peak, consistent with the two proteins interacting as a heterodimeric complex.
Figure 2.
T. castaneumJH receptoris predominantly a heterodimer ofT. castaneummethoprene-tolerantand TcTAI in solution.A, SEC–MALLS analysis of the T. castaneum JH receptor complex (loading concentration of ∼5 mg/ml) showing protein concentration in refractive index units (dRI, black line) and the calculated weight-average molecular weight (MW, broken gray line). The observed molecular weight was ∼103.8 ± 2.1 kDa. B, electrophoretic analysis of fractions of the main peak from the SEC–MALLS analysis. T. castaneum methoprene-tolerant has a theoretical mass of 59.7 kDa, whereas TcTAI has a theoretical mass of 42.7 kDa. C, the native MS spectrum of purified recombinant T. castaneum JH receptor complexes shows 20+ to 24+ charge states (4000–5500 m/z range). D, a zoom of the deconvoluted native MS spectrum of the T. castaneum JH receptor complex provides a neutral mass estimate of 102.6 ± 0.5 kDa, closely matching the theoretical mass of a T. castaneum methoprene-tolerant–TcTAI heterodimer. SEC–MALLS, size-exclusion chromatography in combination with multiangle laser-light scattering; TcTAI, T. castaneum taiman.
The theoretical heterodimer mass was also validated by nano electrospray-time of flight MS under native conditions (native MS), with 20+ to 24+ charge states of the native complex detected across the 4000 to 5500 Da mass/charge range (Fig. 2C). A zoom of the neutral mass spectrum confirmed an average mass of 102.6 ± 0.5 kDa of the methoprene-bound T. castaneum JH receptor complex (Fig. 2D).
We observed that dialysis against buffer lacking methoprene led to instability of the complex with the formation of higher molecular weight material within 24 h as monitored by SEC–MALLS (Fig. S2A). Moreover, it was also observed that solutions of FLAG-T. castaneum methoprene-tolerant–His6-TcTAI complex, even in the presence of methoprene, underwent aggregation over a period of days as assessed by dynamic light scattering (Fig. S2B). This was not prevented by the presence of thiol reagents, 10 mM DTT, 10 mM 2-mercaptoethanol, or 20 mM Tris (2-carboxyethyl) phosphine (TCEP). Together, the present results indicate that purified and recombinant T. castaneum JH receptor preparations comprise an initially relatively stable T. castaneum methoprene-tolerant–TcTAI heterodimer species with a tendency to aggregate in solution on standing for periods of more than a day.
JHRE-binding capacity of the purified JHR complexes
By dimerizing, bHLH-PAS partners form bipartite, basic DNA-binding domains that are necessary for these transcription factors to recognize and bind DNA in order to initiate transcription of specific genes (38). To test whether the purified T. castaneum JH receptor and AaJHR complexes retained DNA-binding activity, the proteins were allowed to interact with radiolabeled DNA probes representing three types of JHREs. Two of them derive from JH-response genes Kr-h1 of T. castaneum (50) and ET of A. aegypti (39) and are designated k-JHRE and ET-JHRE, respectively. The third probe carries a consensus binding site for the AaMET–AaTAI complex that was derived from a random oligonucleotide library through repeated cycles of JHR binding, selection, and amplification, and named MET–FISC binding site 1 (MFBS1) (24).
The EMSA data in Figure 3A show that both the T. castaneum methoprene-tolerant–TcTAI and AaMET–AaTAI protein complexes bound the WT T. castaneum k-JHRE sequence but not its mutated version (lanes 1–5). The JHR protein complexes from both species also bound the ET-JHRE and the consensus binding site MFBS1 (Fig. 3A; lanes 6–11). To confirm the specificity of the interaction, we show that excess of the unlabeled k-JHRE DNA but not its mutated version competed with the [32P]-labeled k-JHRE probe for the T. castaneum methoprene-tolerant–TcTAI complex (Fig. 3B; lanes 1–8), whereas the radiolabeled mutant k-JHRE itself could not bind (lanes 9 and 10). Excess of the unlabeled WT k-JHRE but not of the mutant k-JHRE also effectively competed with the binding of the AaMET–AaTAI protein complex to the [32P]-labeled k-JHRE probe (Fig. S3). To test whether JH III was required for T. castaneum JH receptor binding to k-JHRE, we omitted JH III, which was otherwise added at 10 μM concentration to all EMSA reactions. The persisting protein–DNA complex (Fig. 3B; lane 12) suggests that once formed in the presence of methoprene during the expression and purification steps, the T. castaneum methoprene-tolerant–TcTAI dimer is sufficiently stable to display k-JHRE binding in the EMSA without addition of hormone. This result is consistent with previous findings that JH was not necessary for binding of a bacterially expressed A. aegypti MET–TAI complex to DNA (24). The apparent slight decrease in the band intensity in lane 12 relative to lanes 2 and 11 (Fig. 3B) may however reflect some instability of the T. castaneum methoprene-tolerant–TcTAI dimer in the absence of JH III during the assay.
Figure 3.
RecombinantT. castaneumJH receptorand AaJHR protein complexes bind cognate DNA elements.A, the purified T. castaneum JH receptor (Tc) and AaJHR (Aa) proteins were incubated with the radiolabeled DNA probes in the presence of 10 μM JH III; lanes 1, 6, and 9 contained no protein input. The receptor complexes from both species bound to the WT (wt) sequence of k-JHRE (lanes 2 and 3), but they bound poorly to the mutated (mut) version of k-JHRE (lanes 4 and 5). The retarded protein–DNA complexes (arrowheads) were detected upon electrophoresis by autoradiography. B, the T. castaneum JH receptor protein complex was incubated with the k-JHRE DNA probe in the presence of 100-, 500-, and 1000-fold excess of either WT (wt) or mutated (mut) versions of the unlabeled k-JHRE DNA competitor. Lane 1 contained no protein input; lanes 9 and 10 contained radiolabeled mutant version of k-JHRE. JH III (10 μM) was added to all reactions except that in lane 12. The bracket indicates the unbound radiolabeled DNA probes. AaJHR, Aedes aegypti juvenile hormone receptor; JHRE, juvenile hormone response element.
The purified JHR complexes display JH-binding activity
To determine whether the purified JHR protein complexes retained the expected JH-binding function, we adapted a method based on capturing of hormone–receptor protein complexes on GF/C glass fiber filter discs, previously employed in the assay of [3H]-ponasterone A binding to EcR complexes (51). The results of this procedure were comparable to those obtained by dextran-coated charcoal adsorption, a method originally used to assess binding of [3H]-JH III to proteins that specifically bind JH (22, 23, 52). Data in Figure 4A show that the purified JHR proteins produced in Sf9 cells from the baculovirus constructs bound the [3H]-labeled native hormonal ligand, JH III. The JH III-binding capacity of T. castaneum JH receptor was slightly reduced (by 13%; p < 0.05) after treatment with thermosensitive alkaline phosphatase (TSAP) (Fig. 4A), which resulted in dephosphorylation of the protein (Fig. 5). In a separate experiment, we tested the T. castaneum JH receptor protein complex, which was partially purified from Sf9 cell lysates through a single affinity chromatography step, for effects of prolonged storage and freezing/thawing on ligand binding. As shown in Figure 4B, T. castaneum JH receptor retained its hormone-binding activity whether kept for 3 days at 4 °C or thawed after 1 month at −80 °C, albeit the frozen sample lost about 30% of its activity. Background binding in the GF/C-based assay was demonstrated to be low by use of a T. castaneum methoprene-tolerant protein bearing a mutation V297F in its ligand-binding pocket that prevents JH III from binding to the PAS-B domain of T. castaneum methoprene-tolerant (23) (Fig. 4B).
Figure 4.
Recombinant JHR complexes bind JH III.A, the purified T. castaneumJH receptor, either untreated (ctrl) or dephosphorylated (TSAP), AaJHR, and BSA as an inactive control were assessed for the ability to bind [3H]-JH III (assay described in the Experimental procedures section). B, the same assay was applied to the T. castaneum JH receptor complex, partially purified through the Ni-IMAC chromatography, after storage of the protein in elution buffer (20 mM Tris–HCl [pH 7.8], 150 mM NaCl, 250 mM imidazole), supplemented with 1 μM methoprene and 10% glycerol. The samples were either stored refrigerated at 4 °C for 3 days or thawed 1 month after freezing in liquid nitrogen and storage at −80 °C. A T. castaneum JH receptor complex containing T. castaneum methoprene-tolerantV297F, a mutated variant incapable of binding [3H]-JH III (23), was tested in parallel to the T. castaneum JH receptor complex containing functional T. castaneum methoprene-tolerant (WT). Note that while all protein samples were purified in the presence of methoprene, [3H]-JH III binding occurred, likely because the binding affinity of [3H]-JH III to T. castaneum methoprene-tolerant far exceeds that of methoprene (23). The data are mean values; error bars represent SD; the p values are calculated by t test. AaJHR, Aedes aegypti JHR; BSA, bovine serum albumin; IMAC, immobilized metal affinity chromatography; JHR, juvenile hormone receptor; TSAP, thermosensitive alkaline phosphatase.
Figure 5.
LC–ESI–MS of recombinant purifiedT. castaneumJH receptor complexes under denaturing conditions.A, a zoom at the deconvoluted mass spectrum of FLAG-T. castaneum methoprene-tolerant shows a series of +80 Da mass additions consistent with up to six phosphorylation events (+1-6xPhos) per molecule. B, the major mass of His6-TcTAI (42617.2 Da) matches the theoretical mass with loss of the N-terminal methionine (–Met1 = −131 Da) and additional masses indicating acetylation (+Ac = +42 Da), phosphorylation (+80 Da), and a combination thereof (+Ac+Phos = +122 Da). C and D, treatment of purified T. castaneum JH receptor with thermosensitive alkaline phosphatase (TSAP) largely removed phosphoryl groups from FLAG-T. castaneum methoprene-tolerant and effectively removed them from His6-TcTAI with associated shift in the retention time of the dephosphorylated proteins. Acetylation of His6-TcTAI is not affected, and up to two phosphorylation sites on FLAG-T. castaneum methoprene-tolerant appear to be partially resistant to TSAP treatment under the conditions (see the Experimental procedures section). ESI, electrospray ionization; TcTAI, T. castaneum taiman; aTSAP, thermosensitive alkaline phosphatase.
Distribution and identity of phosphorylation sites of the T. castaneum JH receptor complex
To resolve post-translational modifications and study the composition of T. castaneum JH receptor complexes in more detail, we analyzed the intact mass of T. castaneum methoprene-tolerant and TcTAI subunits under denaturing conditions using LC–electrospray ionization (ESI)–MS. The deconvoluted mass spectrum of the FLAG-T. castaneum methoprene-tolerant subunit showed a series of +80 Da mass additions consistent with up to six phosphorylations per molecule (Fig. 5A). The major mass of the His6-TcTAI subunit indicated the loss of N-terminal methionine with prominent acetylation and phosphorylation modifications (Fig. 5B). Treatment of purified T. castaneum JH receptor with TSAP reduced FLAG-T. castaneum methoprene-tolerant phosphorylation by at least 50%, with an associated shift in the retention time of dephosphorylated proteoforms (Fig. 5, A and C). At least two phosphorylation sites of FLAG-T. castaneum methoprene-tolerant appeared to be resistant to the TSAP treatment (Fig. 5C), which effectively removed phosphorylation of the His6-TcTAI protein (Fig. 5, B and D).
To identify potential phosphorylation sites, we also performed LC–MS/MS analyses of the purified recombinant T. castaneum JH receptor complex. Mascot database searches yielded 53% coverage for FLAG-T. castaneum methoprene-tolerant and 64% sequence coverage for His6-TcTAI, respectively (Table 1). For confident Mascot peptide identifications, see Table S1, annotated phosphopeptide MS/MS evidence spectra are shown in Fig. S4, and corresponding LC–MS/MS data are available from ProteomeXchange under the identifier PXD028394. The analysis uncovered at least ten potentially phosphorylated residues, predominantly clustered in the C-terminal region of FLAG-T. castaneum methoprene-tolerant (Tables 1 and S1; Fig. S4).
Table 1.
Phosphosites identified by shotgun proteomics analysis in the recombinant T. castaneum JH receptor complex
| Protein | Accessiona | Sequence | Molecular weight (Da) | Amino acids | Coverage | Spectrab | Peptidesb |
|---|---|---|---|---|---|---|---|
| FLAG-T. castaneum methoprene-tolerant | A6MUT7 | M1-V516 | 59542.3 | 279/526 | 53% | 51 | 24 |
| Phosphopeptide sequencec | Phosphosites | m/z | Charge | Massd | ΔMass [ppm]e | Scoref | ΔScoref |
|---|---|---|---|---|---|---|---|
| VYGNVNENQDCSTPT(ph)ENSPTKPYYK | T438 | 995.76 | 3 | 2984.254 | 0.16 | 23.6 | 1.9 |
| VYGNVNENQDCSTPTENS(ph)PTKPYYK | S441 | 747.07 | 4 | 2984.247 | −2.19 | 50.9 | 8.6 |
| RPPS(ph)TELGTNIYTSSK | S459 | 610.96 | 3 | 1829.847 | −5.15 | 74.2 | 7.9 |
| RPPST(ph)ELGTNIYTSSK | T460 | 610.96 | 3 | 1829.845 | −5.89 | 71.3 | 4.7 |
| QRTS(ph)PQLSPMSSLPPYPNR | S476 | 746.02 | 3 | 2235.050 | −0.21 | 32.6 | 1.1 |
| TSPQLS(ph)PMSSLPPYPNR | S480 | 976.45 | 2 | 1950.891 | −0.10 | 79.2 | 34.7 |
| TSPQLS(ph)PM(ox)SSLPPYPNR | S480 | 656.63 | 3 | 1966.880 | −3.34 | 30.2 | 3.0 |
| QRTSPQLSPM(ox)S(ph)S(ph)LPPYPNR | S503, S504 | 778.02 | 3 | 2331.024 | 5.27 | 42.1 | 4.1 |
| Protein | Accessiona | Sequence | Molecular weight (Da) | Amino acids | Coverage | Spectrab | Peptidesb |
|---|---|---|---|---|---|---|---|
| His6-TcTAI | S6B9A5 | M42-A411 | 42757.9 | 224/380 | 64% | 36 | 19 |
| Phosphopeptide sequencec | Phosphosites | m/z | Charge | Massd | ΔMass (ppm)e | Scoref | ΔScoref |
|---|---|---|---|---|---|---|---|
| KKS(ph)ETKPQAQINK | S56 | 395.7105 | 4 | 2331.024 | −0.16 | 37.7 | 1.3 |
UniProt identifier.
Numbers of unique spectra and unique peptides, respectively.
S(ph) and T(ph), phosphorylation sites; M(ox), oxidized methionine residues.
Observed precursor mass.
Mass error in parts per million.
Mascot score and Mascot delta score, respectively.
Methoprene-dependent phosphorylation of T. castaneum methoprene-tolerant and its protein interactions
In order to determine if methoprene directly or indirectly mediates T. castaneum methoprene-tolerant phosphorylation and/or regulates stable protein interactions of the T. castaneum JH receptor, we designed the quantitative proteomics experiment illustrated in Figure 6A. Briefly, anti-FLAG pull downs of T. castaneum JH receptor complexes expressed in baculovirus-infected Sf9 cells in the presence or the absence of 5 μM methoprene were prepared. A 1:1 mixture of tryptic digests generated from pull downs of “heavy” (H; +32 Da)-labeled, methoprene-supplemented, or “light” (L; +28 Da)-labeled methoprene-free cell cultures was then analyzed by nano-flow UHPLC–ESI–MS/MS on an Orbitrap Lumos mass spectrometer, as detailed in the Experimental procedures section. MaxQuant database searches confidently identified (false discovery rate [FDR] <1%) several proteins that were significantly enriched (H/L ratio < 0.5; p < 0.05) in pull downs prepared in the absence of methoprene (Fig. 6B, red and Table S2). With FLAG-T. castaneum methoprene-tolerant serving as an internal control with a normalized H/L ratio of 1:1, these included Sf9 homologs of the multimeric heat shock protein HSP90 chaperone complex, namely HSP83 (UniProt: Q9GQG6), HSP70A1 (UniProt: A0A0K2CTM7), and a DnaJ/HSP40-like protein (UniProt: V5ND41). By contrast, the only protein significantly enriched (H/L ratio > 2; p < 0.05) in pull downs with methoprene was His6-TcTAI (Fig. 6B, cyan and Table S2). The over twofold enrichment of His6-TcTAI was confirmed using immunoblots of anti-FLAG pull downs (Fig. 6C). Sf9 host cell proteins that could be detected at similar levels in both anti-FLAG pull downs (Fig. 6B, gray) or could not be reliably quantified are listed in Table S2. Together, these results demonstrate that the addition of the JHR agonist methoprene to growth media reduces interaction(s) of T. castaneum methoprene-tolerant with the host cell HSP83 chaperone complex and promotes the interaction between T. castaneum methoprene-tolerant and TcTAI. This results in an overall increase in the yield and stability of purified T. castaneum JH receptor receptor complexes in the presence of methoprene.
Figure 6.
Affinity-MS and quantitative phosphoproteomics analysis ofT. castaneumJH receptorcomplexes.A, quantitative proteomics workflow to identify methoprene-dependent protein interactions and phosphorylation sites of T. castaneum JH receptor using a stable isotope dimethyl labeling approach. Anti-FLAG pull downs of T. castaneum JH receptor complexes expressed in baculovirus-infected Sf9 cells in the presence (+) or the absence (−) of methoprene were prepared as detailed in the Experimental procedures section. A 1:1 mixture of tryptic peptides of T. castaneum JH receptor pull downs prepared from “heavy” (H; +32 Da)-labeled methoprene-supplemented or “light” (L; +28 Da)-labeled methoprene-free cultures was generated, and a TiO2-enriched phosphopeptide sample was prepared and analyzed by UHPLC–ESI–MS/MS on an Orbitrap Fusion Lumos Tribrid mass spectrometer. MaxQuant database searches facilitated confident identification, phosphosite localization, and quantification of proteins or peptides as detailed in the Experimental procedures section. B, a volcano plot shows a greater than twofold enrichment of His6-TcTAI associated with FLAG-T. castaneum methoprene-tolerant in the presence of methoprene. By contrast, methoprene seems to promote the dissociation of some Sf9 proteins including HSP83, HSP70-1, and a DnaJ/HSP40-like protein (see Table S2 for details). C, immunoblots show roughly equivalent levels of FLAG-T. castaneum methoprene-tolerant present in input (lane 1), unbound (lane 2), void (lane 3), and elution fractions (lanes 4 and 5) in the presence (+) or the absence (−) of methoprene. The same immunoblots reprobed with HRP-conjugated anti-His6 antibody show a significant reduction in the amount of copurified His6-TcTAI in the absence of methoprene (lanes 4 and 5, anti-His6-HRP). ESI, electrospray ionization; HRP, horseradish peroxidase; TcTAI, T. castaneum taiman.
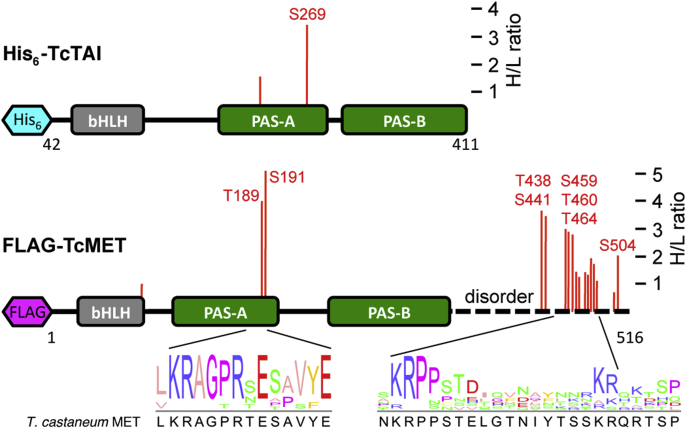
To determine the effect of methoprene on the phosphorylation state of affinity-purified T. castaneum JH receptor complexes, we also performed quantitative proteomics analyses of TiO2-enriched phosphopeptide fractions (Fig. 6A), as detailed in the Experimental procedures section. This approach identified up to 17 possible phosphorylation sites (FDR <1%, location probability >50%) of FLAG-T. castaneum methoprene-tolerant and two of the truncated His6-TcTAI protein (Table 2). Phosphorylation sites of T. castaneum JH receptor complexes mapped to the PAS-A domains of TcTAI (S269) and T. castaneum methoprene-tolerant (T189 and S191) and to a cluster of serine and threonine residues in the C-terminal disordered region of T. castaneum methoprene-tolerant (Table 2 and Fig. 7). Supporting MaxQuant phosphopeptide data are listed in Table S3, and corresponding annotated MS/MS evidence spectra are shown in Fig. S5. The quantitative phosphoproteomics data are available from ProteomeXchange (identifier: PXD028599).
Table 2.
Identification of methoprene-dependent phosphorylation sites in the affinity-purified T. castaneum JH receptor complex
| Protein | Accession | Sequence | Molecular weight (Da) | Amino acids | Coverage | Spectra | Peptides | H/L ratioa |
|---|---|---|---|---|---|---|---|---|
| FLAG-T. castaneum methoprene-tolerant | A6MUT7 | M1-V516 | 59542.3 | 295/526 | 56.0% | 696 | 26 | 0.9 ± 0.4 |
| Phosphopeptide sequence | Phosphositeb | m/z | Charge | ΔMass (ppm) | Scorec | ΔScorec | Probabilityd | H/L ratio |
|---|---|---|---|---|---|---|---|---|
| IYQTLLS(ph)GK | S86 | 579.81 | 2 | −0.06 | 60.5 | 57.8 | 99.6% | 1.0 |
| T(ph)ESAVYEPVR | T189 | 629.79 | 2 | 0.03 | 150.0 | 150.0 | 61.6% | 4.0 |
| TES(ph)AVYEPVR | S191 | 632.81 | 2 | 0.41 | 220.5 | 208.7 | 99.5% | 5.1 |
| VYGNVNENQDCSTPT(ph)ENSPTK | T438 | 1030.16 | 3 | −0.22 | 90.1 | 73.2 | 96.1% | 3.6 |
| VYGNVNENQDCSTPTENS(ph)PTK | S441 | 1024.12 | 3 | 0.72 | 106.4 | 79.3 | 90.1% | 3.4 |
| RPPS(ph)TELGTNIYTSSK | S459 | 943.97 | 2 | −0.01 | 248.5 | 248.5 | 98.4% | 3.0 |
| RPPST(ph)ELGTNIYTSSK | T460 | 944.47 | 2 | −0.25 | 113.5 | 85.3 | 84.6% | 2.9 |
| RPPSTELGT(ph)NIYTSSK | T464 | 633.67 | 3 | −0.44 | 69.0 | 53.9 | 79.8% | 2.8 |
| RPPSTELGTNIYTS(ph)SK | S469 | 629.65 | 3 | −0.10 | 124.2 | 124.2 | 59.4% | 1.5 |
| RPPSTELGTNIYTSS(ph)K | S470 | 629.98 | 3 | 0.02 | 99.1 | 99.1 | 81.6% | 1.3 |
| T(ph)SPQLSPMSSLPPYPNR | T475 | 990.47 | 2 | 0.32 | 298.3 | 270.0 | 100.0% | 1.5 |
| T(ph)SPQLS(ph)PMSSLPPYPNR | T475, S480 | 1033.47 | 2 | 0.24 | 252.0 | 243.2 | 99.5% | 1.9 |
| QRTS(ph)PQLSPMSSLPPYPNR | S476 | 1136.07 | 2 | −1.11 | 199.9 | 149.9 | 99.5% | 1.4 |
| QRTS(ph)PQLSPMS(ph)SLPPYPNR | S476,S483 | 1176.05 | 2 | 0.04 | 127.9 | 127.9 | 90.1% | 1.7 |
| TSPQLSPM(ox)SS(ph)LPPYPNR | S484 | 998.97 | 2 | −0.08 | 133.5 | 125.2 | 84.9% | 1.1 |
| TQITEVTQQT(ph)EPSIYQHDQLLR | T501 | 913.12 | 3 | 0.04 | 73.3 | 72.6 | 87.5% | 0.7 |
| TQITEVTQQTEPS(ph)IYQHDQLLR | S504 | 913.12 | 3 | 0.10 | 127.4 | 123.7 | 99.3% | 2.0 |
| Protein | Accession | Sequence | Molecular weight (Da) | Amino acids | Coverage | Spectra | Peptides | H/L ratioa |
|---|---|---|---|---|---|---|---|---|
| His6-TcTAI | S6B9A5 | M42-A411 | 42757.9 | 330/380 | 86.8% | 484 | 31 | 2.1 ± 0.7 |
| Phosphopeptide sequence | Phosphositeb | m/z | Charge | ΔMass (ppm) | Scorec | ΔScorec | Probabilityd | H/L ratio |
|---|---|---|---|---|---|---|---|---|
| SRS(ph)FSVR | S227 | 473.73 | 2 | 0.04 | 66.27 | 45.75 | 93.1% | 1.5 |
| DQVS(ph)VSDDDGADSGPYLLCVASR | S269 | 1271.06 | 2 | −0.14 | 126.88 | 126.88 | 95.2% | 3.4 |
Other categories as in Table 1.
Normalized ratio of the heavy to light phosphopeptide label partner.
Phosphorylation sites that are more than twofold enriched in the presence of methoprene (H/L ratio > 2) are underlined.
MaxQuant score and MaxQuant delta score, respectively.
MaxQuant phospho (S/T) site probability (cutoff of >50%).
Figure 7.
Mapping of methoprene-dependent phosphorylation sites on the secondary structure ofT. castaneumJH receptorsubunits. Distribution and H/L ratio of methoprene-dependent phosphorylation sites within the domains of the His6-TcTAI and FLAG-T. castaneum methoprene-tolerant proteins based on quantitative phosphoproteomics data in Table 2. The positions of the phosphorylated residues are indicated by red bars with the height of the bar proportional to the H/L ratio; methoprene-dependent phosphorylation sites with H/L ratio >2 are numbered. A putative bipartite nuclear localization signal is predicted within the intrinsically disordered C-terminal region (broken line) of T. castaneum methoprene-tolerant. The diagrams at the bottom show conservation of the sequence contexts around the C-terminal region and PAS-A phosphorylated residues among nine beetle species (see Fig. S6A for details). TcTAI, T. castaneum taiman.
Some peptide sequences isolated from this disordered region contained up to five potential phosphorylation sites and were also found to be doubly phosphorylated, making the confident localization and relative quantitation of individual phosphorylation sites more difficult. Based on the calculated H/L ratios in Table 2, the relative intensity of most phosphorylation sites appeared to change little in the presence or the absence of methoprene. However, we were able to identify up to nine distinct phosphorylation sites in the affinity-purified T. castaneum JH receptor preparations that appeared to be more than twofold enriched by the presence of methoprene. These included TcTAI residue S269 and T. castaneum methoprene-tolerant residues T189/S191 in the PAS-A domains, as well as T. castaneum methoprene-tolerant residues S438, S441, S459, T460, T464, and S504, in the disordered C-terminal region (Table 2 and Fig. 7). Some of the phosphorylation sites within the PAS-A domain and the disordered C-terminal region of T. castaneum methoprene-tolerant are conserved in sequence contexts of MET orthologs from other beetle species (Figs. 7 and S6A).
Nuclear localization of T. castaneum methoprene-tolerant
A majority of the identified phosphorylation sites in the C-terminal sequence of T. castaneum methoprene-tolerant straddle a potential bipartite nuclear localization signal (NLS) (Fig. 7), predicted by the cNLS Mapper program (53). We first examined the function of the T. castaneum methoprene-tolerant putative bipartite NLS itself by mutating to alanine the basic lysine and arginine residues that form the predicted basic clusters of the motif; the resulting T. castaneum methoprene-tolerant variants were designated NLS1 and NLS2, respectively (Fig. 8A). The WT and mutant T. castaneum methoprene-tolerant proteins carrying an N-terminal Myc epitope were expressed in human embryonic kidney 293 (HEK293) cells and stained with an anti-Myc antibody. While the WT protein localized to the nucleus, both NLS1 and NLS2 showed a strong predominantly cytoplasmic signal (Fig. 8, B and C), suggesting that both the basic clusters are essential for the NLS function. Quantification of nuclear-to-cytoplasmic ratios of signal intensities in confocal micrographs of multiple individual cells revealed a greater residual nuclear signal in the case of the NLS1 variant relative to NLS2 variant (Fig. 8C). Mutating the distal basic cluster thus appeared to have a stronger impact. Moreover, while treatment with methoprene stimulated a significant shift of both mutated variants to the cell nuclei, the translocation of the NLS1 T. castaneum methoprene-tolerant protein was more pronounced (Fig. 8, B and C). To examine TcMET localization in a homologous system, we utilized a TcA cell line derived from T. castaneum. Although morphology of the beetle cells did not permit signal quantification from confocal images, we were able to corroborate the function of the bipartite NLS and the agonist effect, in this case of JH III, causing partial nuclear import of the NLS1 mutated T. castaneum methoprene-tolerant protein (Fig. S7).
Figure 8.
Effects of mutations and methoprene on the cellular localization ofT. castaneummethoprene-tolerant.A, mutated variants of T. castaneum methoprene-tolerant. Basic residues (KR, in blue) constituting the basic clusters of a bipartite NLS were mutated to A (variants NLS1 and NLS2). Subsets of residues that are subject to methoprene-dependent or independent phosphorylation (in red) were substituted either with alanine (variants 2A, 7A1, and 7A2) or with aspartic acid (2D and 7D). B, the WT T. castaneum methoprene-tolerant or its mutated variants, all carrying an N-terminal Myc epitope, were expressed in transfected HEK293 cells and detected using an anti-Myc antibody (green); DNA was stained with DAPI (magenta). Representative examples of Myc-T. castaneum methoprene-tolerant localization are single confocal slices. The scale bar represents 10 μm and applies to all images. C, ratios of nuclear-to-cytoplasmic Myc-T. castaneum methoprene-tolerant signal; each circle represents average of integrated pixel intensities from six measurements in individual transfected cells. Mutating the phosphorylable residues to alanine had a small but significant effect on reducing the predominance of nuclear localization. Removing either of the basic clusters of the NLS rendered T. castaneum methoprene-tolerant largely cytoplasmic; 1 μM methoprene induced a partial nuclear retention (visible in B) particularly of the NLS1 variant. The data are mean values with error bars representing SD; the p values (t test) signify differences of the indicated pairwise comparisons. DAPI, 4′,6-diamidino-2-phenylindole; HEK293, human embryonic kidney 293 cell line; meth., 1 μM methoprene; NLS, nuclear localization signal.
To see if the loss of phosphorylation or if mimicking its constitutive gain in the C-terminal region of T. castaneum methoprene-tolerant might affect its intracellular localization, we prepared T. castaneum methoprene-tolerant variants 7A1, 7A2, and 7D, in which subsets of seven of the identified serine and threonine phosphorylated residues were replaced either with alanine to mimic phosphorylation loss or with aspartic acid to emulate constitutive phosphorylation (Fig. 8A). All three mutated T. castaneum methoprene-tolerant variants predominantly localized in the nuclei of HEK293 as well as the TcA cells (Figs. 8B and S7), suggesting that unlike loss of the NLS itself, removal of phosphorylation in its proximity did not prevent nuclear import. However, quantification of signal intensities in the HEK293 cell line showed that loss of the phosphorylation sites caused a minor but significant retention of the 7A1 and 7A2 mutant proteins in the cytoplasm (Fig. 8C). By contrast, the localization of the phosphomimetic 7D variant of T. castaneum methoprene-tolerant did not differ from that of the WT protein.
Phosphorylation status of JHR proteins and their JHRE-binding and JH-binding capabilities
To test if dephosphorylation of the purified baculoviral T. castaneum JH receptor protein complex might affect its binding to the JHRE target DNA, we performed the EMSA as described previously except that the protein was incubated with or without lambda serine/threonine protein phosphatase (λPP) prior to the assay. The relatively mild treatment with λPP caused a ∼320 Da reduction in the average molecular weight of T. castaneum JH receptor with little or no effect on the stability of the partially dephosphorylated T. castaneum methoprene-tolerant–TcTAI complex as determined by native MS (Fig. 9A). Although λPP was effective in removing most of the detectable phosphoryl groups from the T. castaneum JH receptor complex, the treatment did not prevent it from interacting with the JHRE (Fig. 9B). Signal intensities of the retarded bands, as quantified from parallel EMSA experiments, did not show a significant decrease upon λPP treatment relative to incubation in the phosphatase buffer alone (Fig. 9C). The purified AaMET–AaTAI complex was likewise capable of binding the JHRE following the treatment with λPP (Fig. S8). These data suggest that dephosphorylation had no major effect on the capacity of the purified recombinant JHR complexes to bind the JHRE DNA.
Figure 9.
DephosphorylatedT. castaneumJH receptorretains the capacity to bind JHRE DNA and juvenile hormone (JH).A, dephosphorylation of the recombinant T. castaneum JH receptor protein complex purified from the Sf9 cells as confirmed through native MS analysis after incubation with buffer alone (control) or with 40 U/μl of lambda protein phosphatase (λPP) for 1 h at 4 °C. Deconvoluted native MS spectra show multiple and partially resolved +80 Da mass additions (+1-8xPhos) to the T. castaneum JH receptor complex (top). λPP treatment caused a shift in mass distribution that is consistent with the effective removal of phospho modifications without affecting the structural integrity of T. castaneum JH receptor complexes (bottom). B, EMSA was performed with the T. castaneum JH receptor complex. Prior to incubation with the k-JHRE DNA, the protein samples were treated with 40 U/μl of λPP or buffer alone (ctrl) for 1 h at 4 °C. Integrity of the proteins following the treatment was verified on a Coomassie-stained SDS-PAGE (bottom). C, quantification of band-integrated densities determined from four parallel assays revealed a nonsignificant reduction of the signal upon λPP treatment. The data are mean values with error bars representing SD; the p value was calculated by t test. D, WT Myc-T. castaneum methoprene-tolerant and its variants with mutated phosphorylable sites (shown in Fig. 8A) were in vitro translated using the T7-coupled rabbit reticulocyte lysate system; control (ctrl) contained the reticulocyte lysate without DNA input. The translation products were verified through immunoblotting with an anti-Myc antibody (inset) and subjected to [3H]-JH III binding assays. The mutations had no significant effect on JH binding. JHRE, juvenile hormone response element.
To examine whether phosphorylation might influence the JH-binding activity of T. castaneum methoprene-tolerant, we employed the aforedescribed mutations 7A1, 7A2, and 7D along with T. castaneum methoprene-tolerant variants 2A and 2D, where the T189 and S191 residues in the PAS-A domain, both phosphorylated in response to methoprene in Sf9 cells, were again replaced with either alanine or aspartic acid (Fig. 8A). All five mutated T. castaneum methoprene-tolerant proteins and the WT control were produced using an in vitro translation system and tested for binding of [3H]-JH III. All the T. castaneum methoprene-tolerant variants appeared to be expressed at comparable levels and were stable for the assay duration based on immunoblotting using their N-terminal Myc epitope (Fig. 9D). Their capacity to bind the radiolabeled hormone was similar to that of the WT protein (Fig. 9D), indicating that the phosphorylation status of the tested residues had no effect on the JH-binding function, which is located in the PAS-B domain of T. castaneum methoprene-tolerant. This result is consistent with our earlier observation that the ability of the T. castaneum JH receptor complex, purified from the baculovirus/Sf9 cell system, to bind [3H]-JH III was little affected by treatment with the alkaline phosphatase TSAP (Fig. 4A).
Discussion
Evidence converging from multiple systems indicates that a complex between the bHLH–PAS proteins MET and TAI forms a functional JHR in insects (21, 23, 25, 33, 39, 40, 42, 44). Here, we report successful high-yield expression and purification of recombinant MET–TAI JHR complexes from cultured insect cells and evidence of their protein subunit stoichiometry and specific binding to JH III and JHREs. We also characterize post-translational modifications of T. castaneum methoprene-tolerant, its protein–protein interactions, and nuclear localization as influenced by the JHR agonist methoprene. Partial purification of recombinant AaMET and AaTAI proteins expressed in Escherichia coli was reported by Li et al. (24), but the more homologous baculovirus-mediated expression in insect cells described here clearly has significant advantages particularly for the study of post-translational modifications and of functional properties influenced by such modifications.
Stoichiometry of the MET–TAI complex
Although MET and TAI are assumed to form a functional JHR heterodimer (39), the stoichiometry has so far not been experimentally validated. Based on SEC–MALLS, the molecular weight of our purified T. castaneum JH receptor complex is 103.8 ± 2.1 kDa (Fig. 2A). This was confirmed by native MS, which detected a stable complex of 102.6 ± 0.5 kDa (Fig. 2, C and D). Comparison with theoretical molecular weights of the FLAG-T. castaneum methoprene-tolerant–His6-TcTAI heterodimer (102.5 kDa), FLAG-T. castaneum methoprene-tolerant homodimer (119.5 kDa), and His6-TcTAI homodimer (85.5 kDa) clearly indicates that our purified recombinant T. castaneum JH receptor is the heterodimer. Under optimized conditions, deconvoluted native MS spectra showed multiple and partially resolved proteoforms exhibiting +80 Da [HPO3] mass additions (+1-8xPhos) to the T. castaneum JH receptor complex (Fig. 9A). This observation is consistent with high-resolution LC–ESI–MS results demonstrating up to six +80 Da mass additions of T. castaneum methoprene-tolerant plus a single phosphorylation of TcTAI (Fig. 5, A and B).
Interaction of MET with TAI, HSP83, and other proteins
Our data showed that the T. castaneum JH receptor complex is stabilized by the presence of the JHR agonist methoprene. First, removal of methoprene by dialysis led to aggregation of the T. castaneum methoprene-tolerant–TcTAI dimer as assessed using SEC–MALLS (Fig. S2A). Second, MS of proteins that had been pulled down with FLAG-T. castaneum methoprene-tolerant from the Sf9 cells identified His6-TcTAI as strongly enriched in the presence of methoprene. This ligand-dependent association between T. castaneum methoprene-tolerant and TcTAI is in agreement with previous demonstrations that native or synthetic JHR agonists including methoprene induced interactions between the JHR subunits, either in coimmunoprecipitation (23) or two-hybrid assays (26, 33, 39, 54, 55).
In addition to TcTAI, T. castaneum methoprene-tolerant interacted with proteins naturally occurring in the Sf9 host cells, most notably the HSP83, orthologous to vertebrate HSP90. The chaperone HSP90/83 is well known to associate with and support function of nuclear hormone receptors such as the glucocorticoid receptor (56) or the D. melanogaster EcR (57). Interestingly, HSP90 is important for the function of the mammalian homolog of MET, the ligand-activated transcription factor of the bHLH–PAS family, aryl hydrocarbon receptor (AHR) (58, 59). Without an agonist ligand, HSP90 binds the bHLH and PAS-B domains of AHR and stabilizes AHR within a transcriptionally inactive cytoplasmic complex (60). In the presence of an activating ligand, HSP90 facilitates nuclear import of the AHR-containing complex (61). Once in the nucleus, liganded AHR dissociates from HSP90 and forms a DNA-binding transcriptionally active heterodimer with its bHLH–PAS partner ARNT (60, 62, 63, 64).
In D. melanogaster, HSP83 has been shown to mediate methoprene-stimulated nuclear import of MET and the transcriptional induction of the Kr-h1 gene (65), suggesting that the JHR–HSP83 interaction is functionally conserved with the homologous AHR–HSP90 mechanism. Surprisingly, He et al. (65) detected HSP83 among proteins bound to a JHRE sequence upstream of Kr-h1 in D. melanogaster cells cultured with methoprene, leading to a model in which HSP83 becomes part of a transcriptionally active DNA-bound complex containing MET. This model is inconsistent with the mechanism of AHR action, in which agonist binding to the PAS-B domain of AHR displaces HSP90, which, therefore, does not participate in the active AHR–ARNT complex (60, 62, 64). In contrast, our data show that when expressed in the Sf9 cells, T. castaneum methoprene-tolerant preferentially associated with HSP83 in the absence of methoprene. When methoprene was added to the cell culture, T. castaneum methoprene-tolerant dissociated from HSP83 and instead dimerized with TcTAI (Fig. 6). Therefore, our results concur with the established mode of AHR action. The proteomics analysis of complexes pulled down with FLAG-T. castaneum methoprene-tolerant from Sf9 cells revealed several additional proteins of interest, albeit most were insufficiently abundant to be quantified with statistical significance (Table S2).
Nuclear import of MET
While the D. melanogaster MET and GCE proteins predominantly reside in cell nuclei when expressed in heterologous cell lines or in insect tissues (22, 25, 65, 66, 67, 68, 69), JHR agonists further enhance their nuclear localization. Both of the fly paralogs contain multiple NLS motifs, which confer nuclear localization of MET and GCE either constitutively or in response to JH (68, 69, 70). However, signals for nuclear import of JHR proteins have not been identified in insects other than D. melanogaster.
We have discovered in the C-terminal portion of T. castaneum methoprene-tolerant, a functional bipartite NLS, in which each of the two clusters of basic residues is required for the predominant nuclear localization of T. castaneum methoprene-tolerant. The nature and position of this motif correspond to a previously described NLS in D. melanogaster GCE (69). In both cases, the sequences match a consensus of the classical bipartite NLS, defined as (K/R)(K/R)X10-12(K/R)3/5, where X indicates any amino acid and (K/R)3/5 represents at least three of either lysine or arginine out of five consecutive residues (71); in this case, 14 residues separate the basic clusters in T. castaneum methoprene-tolerant. Alignment of the T. castaneum NLS with MET proteins from several other beetle species shows that the linker sequence may be shorter (10–12 residues) in some cases (Fig. S6A). While the C-terminal regions of insect MET/GCE proteins show little sequence homology, the cNLS Mapper tool (53) could predict in this region bipartite NLS motifs matching the aforementioned consensus in species representing evolutionarily distant insect orders, including the honeybee (Hymenoptera), the migratory locust (Orthoptera), the German cockroach (Blattodea), or the linden bug (Hemiptera) (Fig. S6B). In contrast, a sequence separating the basic clusters in a potential bipartite NLS of AaMET is double the consensus length. No NLS has been found in the carboxyl terminal region of the D. melanogaster MET protein (68), which is derived from the ancestral GCE paralog.
Mutating either one of the basic clusters in T. castaneum methoprene-tolerant rendered the protein predominantly cytoplasmic. Addition of methoprene induced partial relocation to the nucleus, which was more appreciable for the NLS1 mutation affecting the proximal KR cluster, whereas the nuclear-to-cytoplasmic ratio was significantly lower for the NLS2 variant (Fig. 8C). The weaker effect of the NLS1 mutation relative to NLS2 could be explained by residual activity of the mutated NLS whose distal basic cluster KRQRT matches a consensus monopartite NLS sequence K(K/R)X(K/R) (71) as predicted using cNLS Mapper (53).
The C-terminal NLSs reside in proline/serine/threonine-rich regions that are intrinsically disordered in both D. melanogaster GCE (69, 72) and T. castaneum methoprene-tolerant proteins (Fig. 7). Our phosphoproteomics data revealed extensive serine/threonine phosphorylation within the disordered region of T. castaneum methoprene-tolerant. Some of the phosphorylated residues occur in close proximity to the basic clusters of the NLS, suggesting that phosphorylation might modulate the subcellular localization of T. castaneum methoprene-tolerant. While replacement of the identified Ser and Thr residues with the phosphomimetic Asp did not alter the nuclear localization of T. castaneum methoprene-tolerant, the variants lacking subsets of the phosphorylable sites were partially retained in the cytoplasm, although the effect was minor. This situation differs from the case of AHR, where phosphorylation of Ser residues straddling a bipartite NLS, located near the N terminus of AHR, inhibited nuclear import (73). Mammalian cell lines have clearly provided valuable experimental systems for demonstration of NLS activity of insect proteins in the present and other studies (68, 69, 70). However, future investigation of phosphorylation-mediated modulation of NLS activity in more homologous systems may be warranted.
Constitutive and methoprene-induced JHR phosphorylation
JH exerts some of its effects via second-messenger signaling initiated at the cell surface (46, 74, 75). Intriguingly, JH III was shown in A. aegypti to trigger a pathway involving Ca2+/calmodulin-dependent kinase II and a protein kinase C downstream of phospholipase C (48, 76). The components of this pathway were required for JH to induce transcription of the mosquito Kr-h1 and ET genes through the JHR subunits, AaMET and AaTAI, phosphorylated in the presence of JH III at multiple unidentified sites (48). A recent LC–MS/MS-based study has detected seven phosphoserine/phosphothreonine residues in recombinant AaMET, expressed in A. aegypti Aag-2 cells (49). The phosphosites mostly occurred within a disordered region in the C-terminal third of the protein. Four of the identified AaMET residues became phosphorylated upon exposure of the Aag-2 cells to JH III, whereas two phosphoserines were dephosphorylated, and one showed no response to the hormone (49).
Our analyses of the purified recombinant T. castaneum JH receptor complex revealed up to six phosphorylations per molecule of T. castaneum methoprene-tolerant and one in the truncated TcTAI protein (Fig. 5). This multiple phosphorylation was corroborated by native MS data detecting up to eight phosphosites in the T. castaneum JH receptor complex (Fig. 9A). Using shotgun LC–MS/MS, we identified at least ten potential phosphoserine and phosphothreonine residues within the C-terminal disordered region of T. castaneum methoprene-tolerant (Table 1). Quantitative phosphoproteomics of T. castaneum JH receptor pulled down from Sf9 cells indicated that phosphorylation of at least two of the C-terminal serine residues in T. castaneum methoprene-tolerant increased upon exposure of the cells to methoprene. The same experiment revealed two additional, previously undetected, methoprene-induced phosphorylations in the PAS-A domains of both T. castaneum methoprene-tolerant and TcTAI proteins (Fig. 7). While the biological significance of these phosphorylation events remains to be investigated, it is clear that multiple phosphorylation, partly regulated by JHR agonists, is a common feature of MET proteins in T. castaneum and A. aegypti.
A threonine residue (T393) phosphorylated in response to JH III has recently been identified in the PAS-B domain of a MET1 protein from the cotton bollworm, Helicoverpa armigera (HaMET1) (77). Mutation to alanine (T393A) prevented HaMET1 from occupying an upstream region of the Kr-h1 gene and from activating its expression, possibly because of a failure of HaMETT393A to interact with HaTAI (77). Whether this might be a conserved mechanism of JHR regulation remains to be determined. The finding of the single T393 phosphorylation in HaMET1 contrasts with multiple phosphosites, detected through LC–MS/MS analyses in nonhomologous regions of either AaMET (49) or T. castaneum methoprene-tolerant (this study).
Previous work in A. aegypti (48) showed that chemical inhibition or RNAi knockdown of Ca2+/calmodulin-dependent kinase II reduced both the expression of the JH-inducible Kr-h1 and ET genes and occupancy of their regulatory DNA by AaMET and AaTAI. When treated with λPP, nuclear protein extracts from female mosquito abdomens lost their capacity to form a retarded complex with the ET JHRE as assessed by EMSA, apparently suggesting that dephosphorylated AaJHR is incapable of binding its cognate DNA (48). It was therefore surprising to see that in similar EMSA experiments, our purified preparations of recombinant T. castaneum JH receptor and AaJHR complexes bound JHRE DNA even following λPP treatment (Figs. 9B and S8). Our treatment conditions were comparable to those employed by Liu et al. (48) except that we used 40 U/μl rather than 1 U/μl of λPP produced by the same company. Moreover, we have been able to verify dephosphorylation of the T. castaneum JH receptor complex by means of native MS (Fig. 9A). While we cannot exclude a critical role of residual phosphosites in T. castaneum JH receptor that might be inaccessible to λPP, our data strongly suggest that phosphorylation is not intrinsically required for binding of the T. castaneum methoprene-tolerant–TcTAI protein complex to DNA. The apparent discrepancy could be attributed to the fact that Liu et al. (48) worked with nuclear protein extracts from cultured mosquito abdomens, whereas we performed EMSA on defined reaction mixtures containing purified recombinant MET–TAI complexes. Two types of explanation reconciling the results from our laboratory and those of Liu et al. (48) involve potential interactions of JHR with proteins present in nuclear extracts. First, an interaction with a protein preventing the intrinsic ability of JHR to bind JHREs might be disrupted by phosphorylation. Second, an interaction favoring JHRE binding might be enhanced by phosphorylation. Our quantitative proteomics analyses have shown that T. castaneum JH receptor forms complexes with HSP83, HSP70-1, and DnaJ/HSP40-like proteins, all of which dissociate when cells are exposed to methoprene, concomitant with changes in JHR phosphorylation. It may be relevant that Soshilov et al. (64) observed transitional states in ligand-dependent transformation of the AHR into its DNA-binding form, accompanied by release of HSP90 from a transitional AHR-containing complex.
While much remains to be learned about post-translational regulation of JHR proteins, it is becoming clear that phosphorylation, subunit assembly, and cellular localization of JHR complexes play major roles in JH signaling. Our purification of active recombinant JHR protein complexes and their initial characterization pave the way for future functional and mechanistic studies.
Experimental procedures
Construction of baculoviruses for coexpression of recombinant MET and TAI proteins
To achieve coexpression of the two JHR subunits in Spodoptera frugiperda Sf9 cells from a single construct, sequences encoding MET and TAI proteins either of T. castaneum or A. aegypti were cloned into the pFastBac Dual vector (Invitrogen). For the T. castaneum JH receptor, the sequence encoding a region from Met-42 to Ala-411 of TcTAI (National Center for Biotechnology Information [NCBI] accession number: XP_008193629.1) was custom synthesized (GenScript) to match the S. frugiperda codon usage. The synthetic TcTAI sequence was N-terminally fused with a hexahistidine tag and cloned into pFastBac Dual under the polyhedrin promoter. The complementary DNA sequence encompassing the entire T. castaneum methoprene-tolerant open reading frame from Met-1 to Val-516 (NCBI accession number: NP_001092812.1) was isolated from T. castaneum total RNA using reverse-transcription PCR (28), N-terminally fused to a FLAG tag and cloned under the p10 promoter in pFastBac Dual containing the TcTAI sequence. To prepare an AaJHR construct, appropriate DNA sequences were obtained using PCR from AaTAI and AaMET complementary DNA clones, kindly provided by Dr A. Raikhel (University of California, Riverside). A sequence spanning the region from Asn-100 to Glu-495 in AaTAI (NCBI accession number: ABE99837.2) was fused with an N-terminal FLAG tag and cloned under the p10 promoter. A sequence encoding the region from Lys-114 to Ser-977 in AaMET (NCBI accession number: AAX55681.1) was expressed with an N-terminal hexahistidine tag using the polyhedrin promoter.
The pFastBac Dual plasmid constructs containing the T. castaneum JH receptor and AaJHR encoding DNA sequences were transformed into the DH10Bac E. coli strain (Invitrogen) to produce recombinant bacmids. Once positive DH10Bac clones were obtained, bacmid DNA was prepared with the PureLink HiPure plasmid DNA kit (Invitrogen). The recombinant bacmid DNA was transfected into the Sf9 cells using the Cellfectin II reagent (Invitrogen), and P1 viral stocks were generated according to the protocol provided by the system manufacturer.
Virus amplification and insect cell culture
The recombinant baculovirus stocks were amplified by adding 1 ml of the initial P1 virus stock to 20 to 25 ml of a Sf9-II cell culture at 2 × 106 viable cells/ml. The culture was harvested 4 to 5 days postinfection. Cells and cellular debris were removed by centrifugation (1500g, 15 min at 4 °C), and the supernatant sterile filtered using 0.2 μm Nalgene bottle top filters with 2% fetal bovine serum (FBS) added (Sigma). Virus stocks were then stored at 4 °C for up to 3 months. Sf9-II, Sf9-III, and Hi-5 cells (Life Technologies) were used for the production of receptor proteins from various constructs. Cells were incubated in E-flasks using serum-free media (SFM; Life Technologies), SF900-II SFM for Sf9-II and Hi-5 cells or Sf900-III SFM for Sf9-III cells, supplemented with 0.1% Pluronic (F68; Life Technologies) to minimize shear damage to cells. Growth temperature was set to 26.5 °C and shaking speed to 110 to 130 rpm.
Scale-up and protein production
Optimal production conditions (best yield and biological activity) were determined in small-scale (50–100 ml E-flask cultures) experiments. Different cell lines (Sf9-II, Sf9-III, and Hi-5), multiplicity of infections (0.1–3), cell densities (in the range of 2–6 × 106 viable cells/ml) and time of harvest (2–5 days postinfection) were tested, and the best conditions were selected for scale-up. Most often, the optimal scale-up parameters for protein expression were Sf9-III cells at 4 × 106 viable cells/ml, with a multiplicity of infection of 1 and harvest taking place 72 h postinfection. Production scale, 6 to 12 l batches, were generated using Thomson or Corning shaker flasks (catalog no.: 931-116; catalog no.: 431-685) at 26.5 °C with 120 to 130 rpm shaking speed. Culture was supplemented with 1 μM methoprene (isopropyl (2E,4E,7S)-11-methoxy-3,7,11-trimethyldodeca-2,4-dienoate [Apollo Scientific]) at time of infection and a further 4 μM methoprene 2 h prior to harvest. Cultures were harvested by centrifugation (1000g, 10 min at 4 °C), and the pellets were washed twice in cold PBS containing 5 μM methoprene (cells were resuspended and pelleted again with centrifugation as aforementioned). Pellets were processed immediately for purification or snap frozen in liquid nitrogen and then stored at −70 °C.
Purification of the T. castaneum JH receptor complex
Protein purification was achieved by resuspending frozen insect cell pellets in chilled lysis buffer at a ratio of 1 g cell pellet per 5 ml lysis buffer (50 mM Hepes, pH 7.5, 300 mM NaCl, 5 mM imidazole, 1 mM phenylmethylsulfonyl fluoride, complete EDTA-free protease inhibitors [Roche]; one tablet/50 ml lysis buffer), phosphatase inhibitor cocktail (1 mM NaF, 1 mM sodium pyrophosphate, 1 mM sodium tartrate, and 1 mM sodium molybdate), and 10 μM methoprene. Typically, 50 to 80 g of cell pellet were processed at a time. The cell suspension was lysed by two passes through an Emulsiflex-C5 homogenizer (Avestin) operating at 15,000 PSI at 4 °C. The lysate was clarified by centrifugation (25,000g for 30 min at 4 °C).
The supernatant containing the T. castaneum JH receptor complex was purified using a combination of immobilized nickel affinity chromatography (Ni-IMAC) and anti-FLAG affinity chromatography in the presence of 10 μM methoprene; the whole purification was completed within 1 day at 4 °C to minimize proteolysis and oxidation of both receptor subunits. Briefly, the clarified insect cell lysate was incubated with Ni–NTA superflow agarose (Qiagen) previously equilibrated with a binding buffer (50 mM Hepes, pH 7.5, 300 mM NaCl, and 10 μM methoprene) containing 5 mM imidazole in batch binding mode. The column was washed extensively with binding buffer, followed by binding buffer containing an additional 50 mM l-arginine and 50 mM l-glutamine and binding buffer with 30 mM imidazole. T. castaneum JH receptor complex was eluted from the IMAC column using binding buffer containing 400 mM imidazole. Fractions eluting from the Ni-IMAC column and containing T. castaneum JH receptor complex were pooled and incubated with anti-FLAG antibody affinity resin previously equilibrated with PBS containing 10 μM methoprene. This resin was prepared by coupling M2 anti-FLAG monoclonal antibody, a product of the American Type Culture Collection 4E11 cell line listed as 4.00E+11 or HB-9259, to Mini Leak Low agarose resin 2.9 mmol groups per liter (Kementec; catalog no.: 1011L) according to the manufacturer's instructions. The column with bound protein was extensively washed with equilibration buffer before eluting with 0.4 mg/ml FLAG peptide in equilibration buffer.
High losses of purified T. castaneum JH receptor complex were incurred using centrifugal membrane-based concentrators. To overcome this issue, purified T. castaneum JH receptor complex was concentrated using anion exchange chromatography. Briefly, T. castaneum JH receptor complex eluting from the anti-FLAG affinity column was loaded onto a 1-ml HiTRAP Q HP column (GE Lifesciences) equilibrated in 50 mM Tris, pH 8.5, containing 2 mM MgCl2, 10 mM DTT, and 10 μM methoprene, with elution via a sharp 0 to 1.0 M NaCl linear gradient.
SDS-PAGE using 4 to 12% gradient Bis–Tris NuPAGE gels and MES electrophoresis buffer systems (Thermo Fisher Scientific) under reducing conditions was followed by immunoblotting. Antihistidine horseradish peroxidase conjugate (Sigma; A7058) and anti-FLAG M2 horseradish peroxidase conjugate (Sigma; A8592) were used to monitor purification of the T. castaneum JH receptor complex. Immunoblots were developed using the Supersignal Western Pico Plus chemiluminescent substrate (Thermo Fisher Scientific), and images were captured using a Versadoc imaging system (Bio-Rad). The molecular weight was estimated using SeeBlue2 molecular weight standards (Thermo Fisher Scientific).
Anti-FLAG pull downs
For quantitative proteomics identification of methoprene-dependent JHR interaction partners and phosphorylation sites, recombinant FLAG-tagged T. castaneum JH receptor complexes were expressed in insect cells, and anti-FLAG pull downs were prepared. Briefly, baculovirus-infected Sf9-III cells expressing T. castaneum JH receptor were incubated in 50 ml Sf900-III SFM with or without 1 μM methoprene for 70 h plus 4 μM methoprene for another 2 h. The cells were harvested 72 h postinfection, washed twice in ice-cold PBS containing 5 μM methoprene by centrifugation (1000g, 10 min, 4 °C), frozen in liquid nitrogen, and then stored at −70 °C.
To prepare FLAG pull downs, cell pellets of 1 ml were resuspended in 4 ml ice-cold lysis buffer with or without methoprene (25 mM Tris, pH 7.4, 150 mM NaCl, 2 mM KCl, 1 mM PMSF, 1 mM sodium tartrate dibasic, 1 mM sodium molybdate, 1× complete protease inhibitors (Roche), 1× phosphatase inhibitor cocktail, and ±10 μM methoprene). Lysates were prepared by sonication on ice (microtip; 40 Hz, 6× 15 s cycles with 15 s rest between each cycle) and centrifuged to remove insoluble material and cell debris (Beckman JA 25.50 rotor; 18,000 rpm, 30 min, 4 °C). Soluble protein was mixed with 400 μl of a 50% slurry of washed anti-FLAG M2 affinity resin (prepared as described previously) and incubated at 4 °C on a rotating wheel for 1 h. Anti-FLAG beads were centrifuged (2000 rpm, 2 min, 4 °C) to collect unbound supernatant (flow-through) and extensively washed in 4× 10 ml ice-cold lysis buffer ± 10 μM methoprene. FLAG-tagged T. castaneum JH receptor complexes were eluted by stepwise addition of 0.2 ml (E1), 0.4 ml (E2), and 0.4 ml (E3) of lysis buffer containing 0.4 mg/ml FLAG peptide ± 10 μM methoprene. The lysate, starting material, flow-through, and elution fractions were analyzed by Coomassie-stained SDS-PAGE and Western blot (anti-His and anti-FLAG antibodies) for quality control prior to MS.
Native MS
Native mass spectra were acquired with a high-resolution Synapt G2Si mass spectrometer (Waters) equipped with a nano-ESI source in positive ion mode. Protein samples were exchanged into 200 mM ammonium acetate (pH 7.4) buffer using Zeba Spin desalting columns (Thermo Fisher Scientific), and native MS was performed in static infusion mode using coated fused-silica PicoTips (New Objective). To guarantee the ionization effect of the sample solution, the capillary voltage was maintained at 1800 V. The temperature and flow rate of the desolvation gas were maintained at 250 °C and 0.6 l/min, respectively. The nebulizer gas pressure was 6.5 bar. To achieve a favorable desolvation effect and to increase the sensitivity, the trap collision energy was set at 90 eV. Quadrupole frequencies, radiofrequency amplitudes, and transfer times were adjusted to achieve best ion transmission efficiency. Native MS data were acquired at 1 Hz across a mass/charge range of 500 to 10,000 Da, and MS raw data were processed using the Protein Metrics Intact Mass parsimonious charge deconvolution algorithm (78).
Intact mass analysis by LC–MS
The accurate mass of purified recombinant proteins was determined by denaturing LC–MS. Protein samples were spiked with formic acid (FA) to a final concentration of 0.1% (v/v) and separated by reverse-phase LC on an UltiMate 3000 RSLCnano system (Thermo Fisher Scientific) fitted with a 50 × 4.6 mm, 5 μM particle size, 300 Å pore size PLRP-S column (Agilent). Proteins were eluted at a flow rate of 250 μl/min by applying a linear 30 min gradient from 0 to 80% solvent B (mobile phase A: 0.1% [v/v] FA; mobile phase B: 90% [v/v] acetonitrile/0.1% [v/v] FA) using an Apollo II electron spray ion source coupled to a micrOTOF-QII mass spectrometer (Bruker). The instrument was calibrated in positive ion mode using ESI-L Low Concentration Tuning Mix (Agilent), and LC–MS raw data were processed and deconvoluted using the MaxEnt algorithm as part of Bruker Compass Data Analysis, version 4.3.
Sample preparation for MS and UPLC–MS/MS phosphoproteomics analysis
For peptide sequencing, affinity MS, and quantitative phosphoproteomics analyses, purified recombinant JHR complexes or anti-FLAG pull downs were digested using a single-pot solid phase–enhanced sample preparation method (79). Protein samples (50–100 μg) were reduced with 5 mM TCEP (Sigma) for 15 min, alkylated for 30 min with 50 mM iodoacetamide (Sigma), and digested with 1 μg trypsin gold (Promega) for 16 h at 37 °C. For quantitative proteomics the peptide solutions were desalted using C18 MacroSpin columns (Nest Group) prior to on-column stable isotope dimethyl labeling of differentially treated samples using light (CH2O) or heavy (CD2O) formaldehyde (Sigma), as previously described (80). Light (+28 Da) and heavy (+32 Da) labeled peptide samples were mixed 1:1 in 1% FA and subjected to phosphopeptide enrichment using TopTip TiO2 microspin columns according to the protocol provided by the manufacturer (Glygen). TiO2-bound or TiO2-unbound fractions were resuspended in 10 μl 0.1% FA, and 3-μl aliquots were separated in duplicate using a two-column chromatography setup comprising a PepMap100 C18 20 mm × 75 μm trap and a PepMap C18 500 mm × 75 μm analytical column (Thermo Fisher Scientific). Samples were concentrated on the trap column at 5 μl/min for 5 min and infused into an Orbitrap Fusion Lumos Tribrid mass spectrometer (Thermo Fisher Scientific) at 300 nl/min running 120 min gradients from 99% buffer A (0.1% FA) to 40% buffer B (99% acetonitrile and 0.1% FA) on a Dionex Ultimate 3000 UPLC system (Thermo Fisher Scientific). The Lumos mass spectrometer was operated in a data-dependent mode, automatically switching between the acquisition of a single Orbitrap MS scan (resolution of 120,000) every 3 s and the top-20 multiply charged precursors selected for EThcD fragmentation (maximum fill time of 100 ms; automatic gain control of 5 × 104 with a resolution of 30,000 for Orbitrap MS/MS scans).
Mass spectra database searching
MS/MS database searches for the characterization of purified JHR complexes and identification of peptide sequences were carried out using Mascot, version 2.3 (Matrix Science). Scaffold, version 4.4.3 (Proteome Software) was used to probabilistically validate Mascot (phospho)peptide identifications using the PeptideProphet algorithm (Table S1 and Fig. S4) (81). The shotgun proteomics data have been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the PRIDE partner repository with the dataset identifier PXD028394 (82). Relative quantification of associated proteins and confidently localized phosphorylation sites was accomplished using MaxQuant (version 1.5.3.1) (83). Searches were performed against a custom protein database containing S. frugiperda Swiss-Prot and TrEMBL sequences, recombinant FLAG-T. castaneum methoprene-tolerant, His6-TcTAI, FLAG-AaTAI, and His6-AaMET proteins, as well as common proteomics contaminants (28,148 entries). High-resolution MS/MS data were searched with trypsin cleavage specificity allowing two miscleavage events, carbamidomethylation of cysteine (+57 Da) set as a fixed modification and oxidation of methionine (+16 Da), acetylation (+42 Da) of protein N termini, and phosphorylation (+80 Da) of serine, threonine, or tyrosine as variable modifications. Stable isotope-labeled samples were searched allowing for additional variable light (L; +28 Da) or heavy (H; +32 Da) dimethyl labels on the peptide N termini and lysine residues. The precursor mass tolerance was set to 20 ppm for the first search and 10 ppm for the main search with a maximum FDR of 1.0% set for protein and peptide identifications. To enhance the identification of peptides between samples, the match between runs option was enabled with the precursor match window set to 2 min and an alignment window of 10 min in addition to the requantification module. Quantitative LC–MS/MS experiments were conducted using two biological replicates with five technical replicates for affinity-purified fractions and three technical replicates for phosphopeptide-enriched fractions. A robust permutation test was used to analyze MaxQuant data and evaluate statistically significant differences in the relative abundance of T. castaneum JH receptor-associated proteins or phosphopeptides (84), and only phosphorylation sites with MaxQuant ΔScore >50 and localization probability >50% were considered. The quantitative (phospho)proteomics data have been deposited to the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the PRIDE partner repository with the dataset identifier PXD028599.
SEC–MALLS
FLAG-T. castaneum methoprene-tolerant and His6-TcTAI were coexpressed and purified as described previously. Purified proteins at ∼5 mg/ml were subjected to size-exclusion chromatography using a Superdex 200 10/300 column (GE Healthcare) at a flow rate of 0.5 ml/min in 50 mM Tris, 350 mM NaCl, 10 μM methoprene, 2 mM MgCl2, 100 μM TCEP, pH 8.0, with UV monitoring at 280, 254, and 215 nm. Fractions were collected in aliquots of 0.5 ml. Light scattering and concentration data were collected by in-line miniDawn TREOS MALLS detector and Optilab T-rEX refractometer (Wyatt Technology), respectively. The weight-average molecular weights were calculated using the intensity of the scattered light in combination with the change in refractive index, whereas the protein concentration at the detector was determined by the change in refractive index. UV, MALLS, and differential refractive index data were collected using the ASTRA software (Wyatt Technology). Molecular weight determinations were also done with the ASTRA software according to the Zimmerman model using the standard dn/dc value of 0.1852 ml/g for proteins (85).
EMSA
Three types of JHRE DNA sequences previously shown to bind MET–TAI complexes from T. castaneum and A. aegypti and/or mediate MET–TAI dependent transcriptional activation were employed as double-stranded DNA probes to demonstrate binding of our recombinant JHR complexes. The k-JHRE sequence TCGCCTCCACGTGCCGTTTCA originates from the immediate JH-response gene Kr-h1 of T. castaneum (50). The E-box–like palindrome (in bold type) is recognized by certain bHLH–PAS proteins. A mutated sequence TCGCCTCACATGTCCGTTTCA lacking this core motif served as a negative control. A second element CCATCCCACACGCGAAGACGATAAAACCA whose core contains an imperfect palindrome (bold) was identified as an enhancer of the JH-inducible ET gene of A. aegypti (39). We refer to this sequence as ET-JHRE. Finally, we used a probe GCCGCACGTGTCGTTGG that again contained the CACGTG palindrome and which was defined as a consensus binding site (called MFBS1) for the A. aegypti MET–TAI complex in an unbiased oligonucleotide screen (24). We added a “T” to the 5′ end of each of the single-stranded oligonucleotides, so that radioactive labeling could be achieved after annealing of the complementary strands using a fill-in reaction with the large Klenow fragment of DNA polymerase and α-[32P]dATP.
The assay was performed with the purified T. castaneum JH receptor and AaJHR proteins as previously described for a MET–TAI complex from A. aegypti (24) with minor modifications. When the JHR complexes were to be dephosphorylated, the protein was treated with 40 U/μl of λPP (New England Biolabs) for 1 h at 4 °C prior to the assay. The EMSA was set up with 250 ng of a JHR protein complex in reactions of 20 μl containing 20 mM Tris–HCl (pH 7.8), 50 mM NaCl, 5 mM MgCl2, 5 mM DTT, 10 μM JH III (Sigma), 10% glycerol, 500 ng/μl bovine serum albumin (BSA), 50 ng/μl of poly(dA-dT), and any unlabeled DNA competitor. After 10 min at room temperature, a [32P]-labeled double-stranded oligonucleotide probe (20 fmol) was added, and the reactions were incubated for a further 20 min. The protein–DNA complexes were then resolved by electrophoresis on a 6% polyacrylamide gel in Tris-borate-EDTA (pH 8.3), after which the gel was fixed, dried, and processed using the Typhoon Phosphoimager 9410. Band intensities were quantified from the raw phosphoimager data as integrated density using the ImageJ software (the National Institutes of Health).
Site-directed mutagenesis of T. castaneum methoprene-tolerant
The overlap extension PCR method (86) was employed to generate T. castaneum methoprene-tolerant variants lacking the putative C-terminal region bipartite NLS by substituting with alanine the pairs of basic residues on either end of the motif: K455A;R456A (NLS1) and K471A;R472A (NLS2), respectively (Fig. 8A). The same method was used to replace the potentially phosphorylated T189 and S191 residues identified in the PAS-A domain with alanine or aspartic acid (variants 2A and 2D, respectively). In all cases, PCR with primers introducing the mutations was performed on a template encoding the entire WT T. castaneum methoprene-tolerant in a pK-Myc-C2 plasmid, and each mutated product was verified by sequencing. For T. castaneum methoprene-tolerant variants 7A1, 7A2, and 7D with overlapping sets of seven serine/threonine residues near the C terminus simultaneously mutated to alanine or aspartic (Fig. 8A), the coding DNA was custom synthesized (GenScript).
[3H]-JH III binding assay
Purified recombinant JHR proteins were assayed for the ability to bind [3H]-JH III (PerkinElmer) essentially as described (23). However, instead of dextran-coated charcoal being used to distinguish between free and protein-bound [3H]-JH III, the hormone–protein complex was captured on GF/C glass fiber discs (Whatman) as routinely employed in our laboratory to monitor [3H]-ponasterone A binding to EcRs (51). Assays were performed in PEG 20,000 coated 6 × 50 mm glass tubes. Binding buffer contained 20 mM Tris (pH 7.8), 5 mM Mg acetate, 1 mM EDTA disodium salt, and 1 mM DTT. [3H]-JH III was freshly prepared in aqueous solution by evaporating [3H]-JH III stored in hexane and replacing the solvent with cold binding buffer to give approximately 20,000 dpm in 15 μl per assay. JHR proteins were diluted in cold binding buffer containing 0.1% BSA to give a final concentration of 0.3 μM. Assays were incubated at room temperature for 30 min. Each assay was applied to a Whatman GF/C glass fiber filter disc and allowed to adsorb for 30 s before transferring to a glass sinter assembly under vacuum and washing with 15 ml ice-cold binding buffer (without BSA). Each filter was transferred to a scintillation vial, and 7 ml InstaGel Plus (Packard TriCarb) scintillation fluid was added. One protein-free control sample did not undergo the washing procedure to record the [3H]-JH III input. Vials were counted for 3 min using a PerkinElmer TRI-CARB 4910 TR liquid scintillation counter. To assess the JH III binding capacity of T. castaneum methoprene-tolerant variants mutated in the potential phosphorylation sites, the coding DNA sequences were cloned behind a T7 RNA polymerase promoter and a Myc epitope in the pK-Myc-C2 vector. The Myc-tagged T. castaneum methoprene-tolerant proteins were expressed by in vitro transcription and translation using the TnT Quick T7-coupled rabbit reticulocyte lysate system (Promega) (23). The resulting proteins were then tested for [3H]-JH III binding as described previously.
Cell transfection, immunocytochemistry, and microscopy image analysis
To assess the subcellular localization of T. castaneum methoprene-tolerant lacking its predicted NLS or phosphorylation sites, the control and mutated Myc-T. castaneum methoprene-tolerant constructs in pK-Myc-C2 were transiently transfected to the HEK293 cell line, where the proteins were expressed under the cytomegalovirus promoter promoter. The cells were grown in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% FBS and were transfected with 1 μg DNA per well in a 6-well plate using the FuGENE HD reagent (Promega). Transfected cells were grown for 36 h on microscope cover slips submersed in the culture wells, after which they were fixed with 4% formaldehyde in PBS, permeabilized with 0.2% Triton X-100, and stained with the 9E10 mouse monoclonal antibody (1:500; Invitrogen) against the Myc epitope, followed by incubation with a secondary antibody coupled with Cy3 (Jackson ImmunoResearch). The samples were mounted in the Vectashield medium (Vector Laboratories), containing 4′,6-diamidino-2-phenylindole for DNA counterstaining. When the effect of a T. castaneum methoprene-tolerant ligand was to be tested, 0.5 μM methoprene in dimethyl sulfoxide was added to the medium 1 h prior to cell fixation. Images were captured using the FluoView FV1000 laser scanning microscope (Olympus) as individual single slices. The relative distribution of Myc-T. castaneum methoprene-tolerant was estimated from the confocal slices of constant thickness by measuring signal intensities within the nuclear and cytoplasmic areas of the transfected HEK293 cells. The data were recorded using the ImageJ software (the National Institutes of Health) as mean gray values, and ratios of nuclear-to-cytoplasmic signal intensity were calculated for a number of individual cells from single confocal slices of constant thickness. To examine T. castaneum methoprene-tolerant localization in cells derived from Tc, Myc-T. castaneum methoprene-tolerant was expressed from the pIEx-4 vector (Novagen) in the TcA cell line (87). TcA cells were grown at 27 °C in the Ex-CELL 420 SFM (Sigma) supplemented with 10% FBS, transiently transfected, and subjected to immunostaining as described previously for the HEK293 cell line.
Data availability
The MS data can be obtained from the PRIDE repository. The Mascot dataset and the associated LC–MS/MS raw data and database search results are accessible under project identifier PXD028394 (LC–MS/MS analyses of purified and recombinant T. castaneum JH receptor complexes). The MaxQuant dataset and the associated isotopically labeled phosphopeptide-enriched LC–MS/MS raw data are accessible under project identifier PXD028599 (quantitative proteomics analysis of affinity-purified T. castaneum JH receptor complexes).
Dedication
This communication is dedicated to Thomas Gordon Wilson 1946 to 2017.
Supporting information
This article contains supporting information.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
We are grateful to Petr Man and Petr Pompach for their generous help with native MS, performed at the Czech Infrastructure for Integrative Structural Biology, and funded by MEYS, Czechia (project number: LM2018127). We also thank the Melbourne Mass Spectrometry and Proteomics Facility of The Bio21 Molecular Science and Biotechnology Institute at the University of Melbourne for the support of MS analysis. Initial mapping of phosphorylation sites by the Australian Proteome Analysis Facility, Macquarie University, North Ryde, is gratefully acknowledged. The research in Commonwealth Scientific and Industrial Research Organisation was enabled by support from the Advanced Materials Transformation Capability Platform directed by Cathy Foley.
Author contributions
M. J., W. J. M., T. N., J. P. M., and R. J. H. conceptualization; M. J., W. J. M., T. N., L. B., B. R., J. S., T. P., L. L., J. K. K. L., L. G. S., G. O. L., and R. J. H. methodology; M. J., T. N., and R. J. H. validation; M. J., W. J. M., T. N., L. B., J. P. M., L. G. S., and R. J. H. formal analysis; M. J., W. J. M., T. N., L. B., J. K. K. L., J. P. M., L. G. S., and R. J. H. investigation; M. J. and R. J. H. resources; M. J. and T. N. data curation; M. J., W. J. M., T. N., J. P. M., and R. J. H. writing–original draft; M. J., T. N., L. B., and R. J. H. writing–review and editing; M. J. and T. N. visualization; M. J., J. P. M., G. O. L., and R. J. H. supervision; M. J. and R. J. H. project administration; M. J. and R. J. H. funding acquisition.
Funding and additional information
The work was supported by the Czech Science Foundation project 20-05151X (to M. J.) and individual fellowships from the Marie Sklodowska-Curie Actions of the European Union, grant agreements 276569 (to M. J.) and 708832 (to L. B.).
Edited by Joseph Jez
Contributor Information
Marek Jindra, Email: jindra@entu.cas.cz.
Ronald J. Hill, Email: ronald.hill@sydney.edu.au.
Supporting information
References
- 1.Jindra M., Palli S.R., Riddiford L.M. The juvenile hormone signaling pathway in insect development. Annu. Rev. Entomol. 2013;58:181–204. doi: 10.1146/annurev-ento-120811-153700. [DOI] [PubMed] [Google Scholar]
- 2.Yamanaka N., Rewitz K.F., O'Connor M.B. Ecdysone control of developmental transitions: Lessons from Drosophila research. Annu. Rev. Entomol. 2013;58:497–516. doi: 10.1146/annurev-ento-120811-153608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nijhout H. Princeton University Press; Princeton, NJ: 1994. Insect Hormones. [Google Scholar]
- 4.Flatt T., Tu M.-P., Tatar M. Hormonal pleiotropy and the juvenile hormone regulation of Drosophila development and life history. Bioessays. 2005;27:999–1010. doi: 10.1002/bies.20290. [DOI] [PubMed] [Google Scholar]
- 5.Roy S., Saha T.T., Zou Z., Raikhel A.S. Regulatory pathways controlling female insect reproduction. Annu. Rev. Entomol. 2018;63:489–511. doi: 10.1146/annurev-ento-020117-043258. [DOI] [PubMed] [Google Scholar]
- 6.Santos C.G., Humann F.C., Hartfelder K. Juvenile hormone signaling in insect oogenesis. Curr. Opin. Insect Sci. 2019;31:43–48. doi: 10.1016/j.cois.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Bellés X. Elsevier Inc; London: 2020. Insect Metamorphosis: From Natural History to Regulation of Development and Evolution. [Google Scholar]
- 8.Koelle M.R., Talbot W.S., Segraves W.A., Bender M.T., Cherbas P., Hogness D.S. The Drosophila EcR gene encodes an ecdysone receptor, a new member of the steroid receptor superfamily. Cell. 1991;67:59–77. doi: 10.1016/0092-8674(91)90572-g. [DOI] [PubMed] [Google Scholar]
- 9.Thomas H.E., Stunnenberg H.G., Stewart A.F. Heterodimerization of the Drosophila ecdysone receptor with retinoid X receptor and ultraspiracle. Nature. 1993;362:471–475. doi: 10.1038/362471a0. [DOI] [PubMed] [Google Scholar]
- 10.Yao T.P., Forman B.M., Jiang Z., Cherbas L., Chen J.D., McKeown M., Cherbas P., Evans R.M. Functional ecdysone receptor is the product of EcR and ultraspiracle genes. Nature. 1993;366:476–479. doi: 10.1038/366476a0. [DOI] [PubMed] [Google Scholar]
- 11.Hill R.J., Billas I.M.L., Bonneton F., Graham L.D., Lawrence M.C. Ecdysone receptors: From the Ashburner model to structural biology. Annu. Rev. Entomol. 2013;58:251–271. doi: 10.1146/annurev-ento-120811-153610. [DOI] [PubMed] [Google Scholar]
- 12.Hill R.J., Graham L.D., Turner K.A., Howell L., Tohidi-Esfahani D., Fernley R., Grusovin J., Ren B., Pilling P., Lu L., Phan T., Pollard G.O.L., Pawlak-Skrzecz A., Streltsov V.A., Peat T.S., et al. Structure and function of ecdysone receptors-interactions with ecdysteroids and synthetic agonists. Adv. Insect Physiol. 2012;43:299–351. [Google Scholar]
- 13.Riddiford L.M. Rhodnius, golden oil, and Met: A history of juvenile hormone research. Front. Cell Dev. Biol. 2020;8:679. doi: 10.3389/fcell.2020.00679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones G., Jones D., Li X., Tang L., Ye L., Teal P., Riddiford L., Sandifer C., Borovsky D., Martin J.-R. Activities of natural methyl farnesoids on pupariation and metamorphosis of Drosophila melanogaster. J. Insect Physiol. 2010;56:1456–1464. doi: 10.1016/j.jinsphys.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Billas I.M.L., Iwema T., Garnier J.-M., Mitschler A., Rochel N., Moras D. Structural adaptability in the ligand-binding pocket of the ecdysone hormone receptor. Nature. 2003;426:91–96. doi: 10.1038/nature02112. [DOI] [PubMed] [Google Scholar]
- 16.Carmichael J.A. The X-ray structure of a hemipteran ecdysone receptor ligand-binding domain: Comparison with a lepidopteran ecdysone receptor ligand-binding domain and implications for insecticide design. J. Biol. Chem. 2005;280:22258–22269. doi: 10.1074/jbc.M500661200. [DOI] [PubMed] [Google Scholar]
- 17.Ren B., Peat T.S., Streltsov V.A., Pollard M., Fernley R., Grusovin J., Seabrook S., Pilling P., Phan T., Lu L., Lovrecz G.O., Graham L.D., Hill R.J. Unprecedented conformational flexibility revealed in the ligand-binding domains of the Bovicola ovis ecdysone receptor (EcR) and ultraspiracle (USP) subunits. Acta Crystallogr. D Biol. Crystallogr. 2014;70:1954–1964. doi: 10.1107/S1399004714009626. [DOI] [PubMed] [Google Scholar]
- 18.Wilson T.G., Fabian J. A Drosophila melanogaster mutant resistant to a chemical analog of juvenile hormone. Dev. Biol. 1986;118:190–201. doi: 10.1016/0012-1606(86)90087-4. [DOI] [PubMed] [Google Scholar]
- 19.Wilson T.G., Ashok M. Insecticide resistance resulting from an absence of target-site gene product. Proc. Natl. Acad. Sci. U. S. A. 1998;95:14040–14044. doi: 10.1073/pnas.95.24.14040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore A.W., Barbel S., Jan L.Y., Jan Y.N. A genomewide survey of basic helix-loop-helix factors in Drosophila. Proc. Natl. Acad. Sci. U. S. A. 2000;97:10436–10441. doi: 10.1073/pnas.170301897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jindra M., Tumova S., Milacek M., Bittova L. A decade with the juvenile hormone receptor. Adv. Insect Physiol. 2021;60:37–85. [Google Scholar]
- 22.Miura K., Oda M., Makita S., Chinzei Y. Characterization of the Drosophila methoprene-tolerantgene product. FEBS J. 2005;272:1169–1178. doi: 10.1111/j.1742-4658.2005.04552.x. [DOI] [PubMed] [Google Scholar]
- 23.Charles J.-P., Iwema T., Epa V.C., Takaki K., Rynes J., Jindra M. Ligand-binding properties of a juvenile hormone receptor, Methoprene-tolerant. Proc. Natl. Acad. Sci. U. S. A. 2011;108:21128–21133. doi: 10.1073/pnas.1116123109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li M., Liu P., Wiley J.D., Ojani R., Bevan D.R., Li J., Zhu J. A steroid receptor coactivator acts as the DNA-binding partner of the methoprene-tolerant protein in regulating juvenile hormone response genes. Mol. Cell. Endocrinol. 2014;394:47–58. doi: 10.1016/j.mce.2014.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jindra M., Uhlirova M., Charles J.-P., Smykal V., Hill R.J. Genetic evidence for function of the bHLH-PAS protein Gce/Met as a juvenile hormone receptor. PLoS Genet. 2015;11 doi: 10.1371/journal.pgen.1005394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bittova L., Jedlička P., Dracinsky M., Kirubakaran P., Vondrasek J., Hanus R., Jindra M. Exquisite ligand stereoselectivity of a Drosophila juvenile hormone receptor contrasts with its broad agonist repertoire. J. Biol. Chem. 2019;294:410–423. doi: 10.1074/jbc.RA118.005992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdou M.A., He Q., Wen D., Zyaan O., Wang J., Xu J., Baumann A.A., Joseph J., Wilson T.G., Li S., Wang J. Drosophila Met and Gce are partially redundant in transducing juvenile hormone action. Insect Biochem. Mol. Biol. 2011;41:938–945. doi: 10.1016/j.ibmb.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Konopova B., Jindra M. Juvenile hormone resistance gene Methoprene-tolerant controls entry into metamorphosis in the beetle Tribolium castaneum. Proc. Natl. Acad. Sci. U. S. A. 2007;104:10488–10493. doi: 10.1073/pnas.0703719104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Konopova B., Smykal V., Jindra M. Common and distinct roles of juvenile hormone signaling genes in metamorphosis of holometabolous and hemimetabolous insects. PLoS One. 2011;6 doi: 10.1371/journal.pone.0028728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lozano J., Bellés X. Role of Methoprene-tolerant (Met) in adult morphogenesis and in adult ecdysis of Blattella germanica. PLoS One. 2014;9 doi: 10.1371/journal.pone.0103614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daimon T., Uchibori M., Nakao H., Sezutsu H., Shinoda T. Knockout silkworms reveal a dispensable role for juvenile hormones in holometabolous life cycle. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E4226–E4235. doi: 10.1073/pnas.1506645112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minakuchi C., Namiki T., Shinoda T. Krüppel homolog 1, an early juvenile hormone-response gene downstream of Methoprene-tolerant, mediates its anti-metamorphic action in the red flour beetle Tribolium castaneum. Dev. Biol. 2009;325:341–350. doi: 10.1016/j.ydbio.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 33.Kayukawa T., Minakuchi C., Namiki T., Togawa T., Yoshiyama M., Kamimura M., Mita K., Imanishi S., Kiuchi M., Ishikawa Y., Shinoda T. Transcriptional regulation of juvenile hormone-mediated induction of Krüppel homolog 1, a repressor of insect metamorphosis. Proc. Natl. Acad. Sci. U. S. A. 2012;109:11729–11734. doi: 10.1073/pnas.1204951109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kayukawa T., Jouraku A., Ito Y., Shinoda T. Molecular mechanism underlying juvenile hormone-mediated repression of precocious larval-adult metamorphosis. Proc. Natl. Acad. Sci. U. S. A. 2017;114:1057–1062. doi: 10.1073/pnas.1615423114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lozano J., Bellés X. Conserved repressive function of Krüppel homolog 1 on insect metamorphosis in hemimetabolous and holometabolous species. Sci. Rep. 2011;1:163. doi: 10.1038/srep00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jindra M. Where did the pupa come from? The timing of juvenile hormone signalling supports homology between stages of hemimetabolous and holometabolous insects. Phil. Trans. R. Soc. Lond. B Biol. Sci. 2019;374:20190064. doi: 10.1098/rstb.2019.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Truman J.W. The evolution of insect metamorphosis. Curr. Biol. 2019;29:R1252–R1268. doi: 10.1016/j.cub.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 38.Kewley R.J., Whitelaw M.L., Chapman-Smith A. The mammalian basic helix-loop-helix/PAS family of transcriptional regulators. Int. J. Biochem. Cell Biol. 2004;36:189–204. doi: 10.1016/s1357-2725(03)00211-5. [DOI] [PubMed] [Google Scholar]
- 39.Li M., Mead E.A., Zhu J. Heterodimer of two bHLH-PAS proteins mediates juvenile hormone-induced gene expression. Proc. Natl. Acad. Sci. U. S. A. 2011;108:638–643. doi: 10.1073/pnas.1013914108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Z., Xu J., Sheng Z., Sui Y., Palli S.R. Steroid receptor co-activator is required for juvenile hormone signal transduction through a bHLH-PAS transcription factor, methoprene tolerant. J. Biol. Chem. 2011;286:8437–8447. doi: 10.1074/jbc.M110.191684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bai J., Uehara Y., Montell D.J. Regulation of invasive cell behavior by taiman, a Drosophila protein related to AIB1, a steroid receptor coactivator amplified in breast cancer. Cell. 2000;103:1047–1058. doi: 10.1016/s0092-8674(00)00208-7. [DOI] [PubMed] [Google Scholar]
- 42.Zou Z., Saha T.T., Roy S., Shin S.W., Backman T.W.H., Girke T., White K.P., Raikhel A.S. Juvenile hormone and its receptor, methoprene-tolerant, control the dynamics of mosquito gene expression. Proc. Natl. Acad. Sci. U. S. A. 2013;110:E2173–E2181. doi: 10.1073/pnas.1305293110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cui Y., Sui Y., Xu J., Zhu F., Palli S.R. Juvenile hormone regulates Aedes aegypti Krüppel homolog 1 through a conserved E box motif. Insect Biochem. Mol. Biol. 2014;52:23–32. doi: 10.1016/j.ibmb.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lozano J., Kayukawa T., Shinoda T., Bellés X. A role for taiman in insect metamorphosis. PLoS Genet. 2014;10 doi: 10.1371/journal.pgen.1004769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yokoi T., Nabe T., Ishizuka C., Hayashi K., Ito-Harashima S., Yagi T., Nakagawa Y., Miyagawa H. Transcription-inducing activity of natural and synthetic juvenile hormone agonists through the Drosophila Methoprene-tolerant protein. Pest Manag. Sci. 2020;76:2316–2323. doi: 10.1002/ps.5766. [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto K., Chadarevian A., Pellegrini M. Juvenile hormone action mediated in male accessory glands of Drosophila by calcium and kinase C. Science. 1988;239:916–919. doi: 10.1126/science.3124270. [DOI] [PubMed] [Google Scholar]
- 47.Wilson T.G., DeMoor S., Lei J. Juvenile hormone involvement in Drosophila melanogaster male reproduction as suggested by the Methoprene-tolerant27 mutant phenotype. Insect Biochem. Mol. Biol. 2003;33:1167–1175. doi: 10.1016/j.ibmb.2003.06.007. [DOI] [PubMed] [Google Scholar]
- 48.Liu P., Peng H.-J., Zhu J. Juvenile hormone-activated phospholipase C pathway enhances transcriptional activation by the methoprene-tolerant protein. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E1871–E1879. doi: 10.1073/pnas.1423204112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim K., Albishi N.M., Palli S.R. Identification of juvenile hormone-induced posttranslational modifications of methoprene tolerant and Krüppel homolog 1 in the yellow fever mosquito, Aedes aegypti. J. Proteomics. 2021;242:104257. doi: 10.1016/j.jprot.2021.104257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kayukawa T., Tateishi K., Shinoda T. Establishment of a versatile cell line for juvenile hormone signaling analysis in Tribolium castaneum. Sci. Rep. 2013;3:1570. doi: 10.1038/srep01570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Graham L.D., Johnson W.M., Pawlak-Skrzecz A., Eaton R.E., Bliese M., Howell L., Hannan G.N., Hill R.J. Ligand binding by recombinant domains from insect ecdysone receptors. Insect Biochem. Mol. Biol. 2007;37:611–626. doi: 10.1016/j.ibmb.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 52.Touhara K., Lerro K.A., Bonning B.C., Hammock B.D., Prestwich G.D. Ligand binding by a recombinant insect juvenile hormone binding protein. Biochemistry. 1993;32:2068–2075. doi: 10.1021/bi00059a026. [DOI] [PubMed] [Google Scholar]
- 53.Kosugi S., Hasebe M., Tomita M., Yanagawa H. Systematic identification of cell cycle-dependent yeast nucleocytoplasmic shuttling proteins by prediction of composite motifs. Proc. Natl. Acad. Sci. U. S. A. 2009;106:10171–10176. doi: 10.1073/pnas.0900604106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shin S.W., Zou Z., Saha T.T., Raikhel A.S. bHLH-PAS heterodimer of methoprene-tolerant and cycle mediates circadian expression of juvenile hormone-induced mosquito genes. Proc. Natl. Acad. Sci. U. S. A. 2012;109:16576–16581. doi: 10.1073/pnas.1214209109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miyakawa H., Iguchi T. Comparative luciferase assay for establishing reliable in vitro screening system of juvenile hormone agonists. J. Appl. Toxicol. 2017;37:1082–1090. doi: 10.1002/jat.3459. [DOI] [PubMed] [Google Scholar]
- 56.Picard D. Chaperoning steroid hormone action. Trends Endocrinol. Metab. 2006;17:229–235. doi: 10.1016/j.tem.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 57.Arbeitman M.N., Hogness D.S. Molecular chaperones activate the Drosophila ecdysone receptor, an RXR heterodimer. Cell. 2000;101:67–77. doi: 10.1016/S0092-8674(00)80624-8. [DOI] [PubMed] [Google Scholar]
- 58.Pongratz I., Mason G.G., Poellinger L. Dual roles of the 90-kDa heat shock protein hsp90 in modulating functional activities of the dioxin receptor. J. Biol. Chem. 1992;267:13728–13734. [PubMed] [Google Scholar]
- 59.Whitelaw M.L., McGuire J., Picard D., Gustafsson J.A., Poellinger L. Heat shock protein hsp90 regulates dioxin receptor function in vivo. Proc. Natl. Acad. Sci. U. S. A. 1995;92:4437–4441. doi: 10.1073/pnas.92.10.4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Soshilov A., Denison M.S. Ligand displaces heat shock protein 90 from overlapping binding sites within the aryl hydrocarbon receptor ligand-binding domain. J. Biol. Chem. 2011;286:35275–35282. doi: 10.1074/jbc.M111.246439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kazlauskas A., Sundström S., Poellinger L., Pongratz I. The hsp90 chaperone complex regulates intracellular localization of the dioxin receptor. Mol. Cell. Biol. 2001;21:2594–2607. doi: 10.1128/MCB.21.7.2594-2607.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beischlag T.V., Morales J.L., Hollingshead B.D., Perdew G.H. The aryl hydrocarbon receptor complex and the control of gene expression. Crit. Rev. Eukaryot. Gene Expr. 2008;18:207–250. doi: 10.1615/critreveukargeneexpr.v18.i3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Denison M.S., Soshilov A.A., He G., DeGroot D.E., Zhao B. Exactly the same but different: Promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicol. Sci. 2011;124:1–22. doi: 10.1093/toxsci/kfr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soshilov A.A., Motta S., Bonati L., Denison M.S. Transitional states in ligand-dependent transformation of the aryl hydrocarbon receptor into its DNA-binding form. Int. J. Mol. Sci. 2020;21:2474. doi: 10.3390/ijms21072474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.He Q., Wen D., Jia Q., Cui C., Wang J., Palli S.R., Li S. Heat shock protein 83 (Hsp83) facilitates Methoprene-tolerant (Met) nuclear import to modulate juvenile hormone signaling. J. Biol. Chem. 2014;289:27874–27885. doi: 10.1074/jbc.M114.582825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pursley S., Ashok M., Wilson T.G. Intracellular localization and tissue specificity of the Methoprene-tolerant (Met) gene product in Drosophila melanogaster. Insect Biochem. Mol. Biol. 2000;30:839–845. doi: 10.1016/s0965-1748(00)00056-4. [DOI] [PubMed] [Google Scholar]
- 67.He Q., Zhang Y., Zhang X., Xu D., Dong W., Li S., Wu R. Nucleoporin Nup358 facilitates nuclear import of Methoprene-tolerant (Met) in an importin β- and Hsp83-dependent manner. Insect Biochem. Mol. Biol. 2017;81:10–18. doi: 10.1016/j.ibmb.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 68.Greb-Markiewicz B., Orłowski M., Dobrucki J., Ożyhar A. Sequences that direct subcellular traffic of the Drosophila Methoprene-tolerant protein (MET) are located predominantly in the PAS domains. Mol. Cell. Endocrinol. 2011;345:16–26. doi: 10.1016/j.mce.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 69.Greb-Markiewicz B., Sadowska D., Surgut N., Godlewski J., Zarębski M., Ożyhar A. Mapping of the sequences directing localization of the Drosophila germ cell-expressed protein (GCE) PLoS One. 2015;10 doi: 10.1371/journal.pone.0133307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Greb-Markiewicz B., Kolonko M. Subcellular localization signals of bHLH-PAS proteins: Their significance, current state of knowledge and future perspectives. Int. J. Mol. Sci. 2019;20:4746. doi: 10.3390/ijms20194746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lange A., Mills R.E., Lange C.J., Stewart M., Devine S.E., Corbett A.H. Classical nuclear localization signals: Definition, function, and interaction with importin α. J. Biol. Chem. 2007;282:5101–5105. doi: 10.1074/jbc.R600026200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kolonko M., Bystranowska D., Taube M., Kozak M., Bostock M., Popowicz G., Ożyhar A., Greb-Markiewicz B. The intrinsically disordered region of GCE protein adopts a more fixed structure by interacting with the LBD of the nuclear receptor FTZ-F1. Cell Commun. Signal. 2020;18:180. doi: 10.1186/s12964-020-00662-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ikuta T., Kobayashi Y., Kawajiri K. Phosphorylation of nuclear localization signal inhibits the ligand-dependent nuclear import of aryl hydrocarbon receptor. Biochem. Biophys. Res. Commun. 2004;317:545–550. doi: 10.1016/j.bbrc.2004.03.076. [DOI] [PubMed] [Google Scholar]
- 74.Davey K. Hormonal control of the follicular epithelium during vitellogenin uptake. Invertebr. Reprod. Dev. 1996;30:249–254. [Google Scholar]
- 75.Jing Y.-P., An H., Zhang S., Wang N., Zhou S. Protein kinase C mediates juvenile hormone-dependent phosphorylation of Na+/K+-ATPase to induce ovarian follicular patency for yolk protein uptake. J. Biol. Chem. 2018;293:20112–20122. doi: 10.1074/jbc.RA118.005692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ojani R., Liu P., Fu X., Zhu J. Protein kinase C modulates transcriptional activation by the juvenile hormone receptor methoprene-tolerant. Insect Biochem. Mol. Biol. 2016;70:44–52. doi: 10.1016/j.ibmb.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li Y.-X., Wang D., Zhao W.-L., Zhang J.-Y., Kang X.-L., Li Y.-L., Zhao X.-F. Juvenile hormone induces methoprene-tolerant 1 phosphorylation to increase interaction with Taiman in Helicoverpa armigera. Insect Biochem. Mol. Biol. 2021;130:103519. doi: 10.1016/j.ibmb.2021.103519. [DOI] [PubMed] [Google Scholar]
- 78.Bern M., Caval T., Kil Y.J., Tang W., Becker C., Carlson E., Kletter D., Sen K.I., Galy N., Hagemas D., Franc V., Heck A.J.R. Parsimonious charge deconvolution for native mass spectrometry. J. Proteome Res. 2018;17:1216–1226. doi: 10.1021/acs.jproteome.7b00839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hughes C.S., Foehr S., Garfield D.A., Furlong E.E., Steinmetz L.M., Krijgsveld J. Ultrasensitive proteome analysis using paramagnetic bead technology. Mol. Syst. Biol. 2014;10:757. doi: 10.15252/msb.20145625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Boersema P.J., Raijmakers R., Lemeer S., Mohammed S., Heck A.J.R. Multiplex peptide stable isotope dimethyl labeling for quantitative proteomics. Nat. Protoc. 2009;4:484–494. doi: 10.1038/nprot.2009.21. [DOI] [PubMed] [Google Scholar]
- 81.Keller A., Nesvizhskii A.I., Kolker E., Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 2002;74:5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 82.Perez-Riverol Y., Csordas A., Bai J., Bernal-Llinares M., Hewapathirana S., Kundu D.J., Inuganti A., Griss J., Mayer G., Eisenacher M., Pérez E., Uszkoreit J., Pfeuffer J., Sachsenberg T., Yilmaz S., et al. The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 2019;47:D442–D450. doi: 10.1093/nar/gky1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cox J., Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008;26:1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- 84.Chen D., Shah A., Nguyen H., Loo D., Inder K.L., Hill M.M. Online quantitative proteomics p-value calculator for permutation-based statistical testing of peptide ratios. J. Proteome Res. 2014;13:4184–4191. doi: 10.1021/pr500525e. [DOI] [PubMed] [Google Scholar]
- 85.Folta-Stogniew E. In: Nedelkov D., Nelson R.W., editors. Vol 328. Humana Press Inc; Totowa, NJ: 2006. Oligomeric states of proteins determined by size-exclusion chromatography coupled with light scattering, absorbance, and refractive index detectors; pp. 97–112. (New and Emerging Proteomic Techniques). Methods Mol. Biol. [DOI] [PubMed] [Google Scholar]
- 86.Ho S.N., Hunt H.D., Horton R.M., Pullen J.K., Pease L.R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 87.Silver K., Jiang H., Fu J., Phillips T.W., Beeman R.W., Park Y. The Tribolium castaneum cell line TcA: A new tool kit for cell biology. Sci. Rep. 2014;4:6840. doi: 10.1038/srep06840. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The MS data can be obtained from the PRIDE repository. The Mascot dataset and the associated LC–MS/MS raw data and database search results are accessible under project identifier PXD028394 (LC–MS/MS analyses of purified and recombinant T. castaneum JH receptor complexes). The MaxQuant dataset and the associated isotopically labeled phosphopeptide-enriched LC–MS/MS raw data are accessible under project identifier PXD028599 (quantitative proteomics analysis of affinity-purified T. castaneum JH receptor complexes).