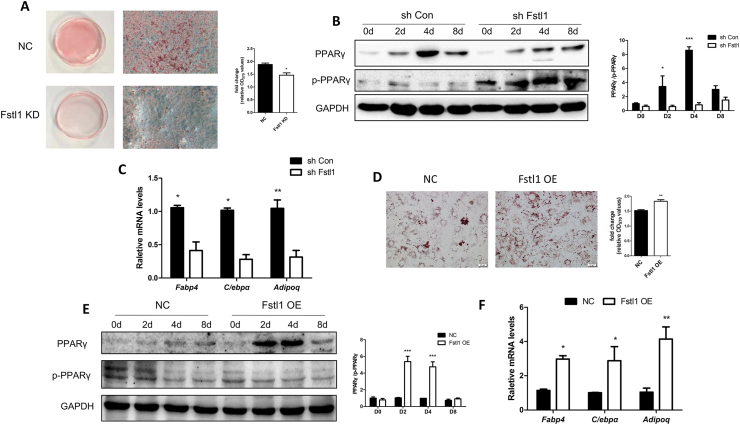
Figure 3.
Differentiation of 3T3L1 cells was inhibited or promoted by Fstl1 knockdown or overexpression. (A) 3T3L1 fibroblasts expressing shFstl1 or vector control were differentiated into adipocytes and stained with Oil Red O at day 8. The oil red O stain was extracted and quantitated by measuring absorbance at 492 nm, Student's t test, n = 3. Scale bar, 100 μm. (B) The protein levels of PPARγ and p-PPARγ during differentiation of the 3T3L1-NC and 3T3L1-shFstl1 groups were detected by western blot. PPARγ and p-PPARγ band intensities were normalized relative to the GAPDH bands, and the PPARγ/p-PPARγ ratio was calculated and analyzed. (C) mRNA levels of adipogenic genes (Fabp4, C/ebpα, Adipoq) in 3T3L1 cells (as in A) were detected by qPCR. Two-way ANOVA followed by Bonferroni post-tests, n = 3. (D) 3T3L1 fibroblasts with Fstl1 overexpression or control were induced for differentiation using cocktail with no rosiglitazone, and stained with Oil Red O at day 8. The oil red O stain was extracted and quantitated by measuring absorbance at 492 nm, n = 3. Scale bar, 100 μm. (E) The protein levels of PPARγ and p-PPARγ during differentiation of 3T3L1 cells with Fstl1 OE and control groups were detected by western blot. The PPARγ/p-PPARγ ratio was analyzed as mentioned above. (F) mRNA levels of adipogenic genes in 3T3L1 cells (as in D) were detected by qPCR. Two-way ANOVA followed by Bonferroni post-tests, n = 3. Data are expressed as mean ± SEM, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.005.