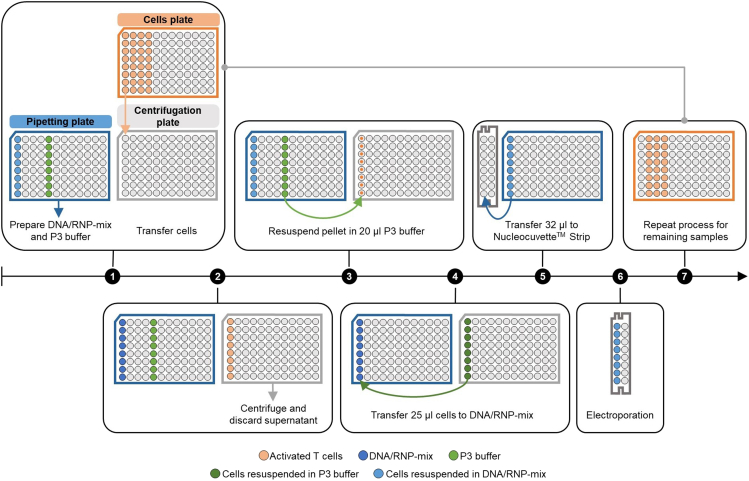
Figure 6.
Workflow of preparing samples for electroporation
Step 1: The targeting construct mix is prepared in the ‘pipetting plate’ (blue plate) by mixing DNA template and RNPs (light blue wells). Additionally, 20 μL P3 buffer is added to the plate (light green wells). Cells to be electroporated are transferred from the ‘cells plate’ (orange plate) to the ‘centrifugation plate’ (grey plate). Step 2: After centrifugation, the supernatant in the ‘centrifugation plate’ is discarded. Step 3: Cell pellets are resuspended in 20 μL of P3 electroporation buffer. Step 4: 25 μL of resuspended cells (dark green) are transferred to the DNA/RNP-mix wells in the ‘pipetting plate’ and mixed. Step 5: 32 μL of cells/DNA/RNPs-mix (dark blue) is transferred to the 16-well NucleocuvetteTM Strip. Step 6: NucleocuvetteTM Strip is placed in the NucleofectorTM and cells are electroporated. Afterwards, cells are transferred to the 24-well plate with pre-warmed SC- medium (not shown). Step 7: Process is repeated for remaining samples.