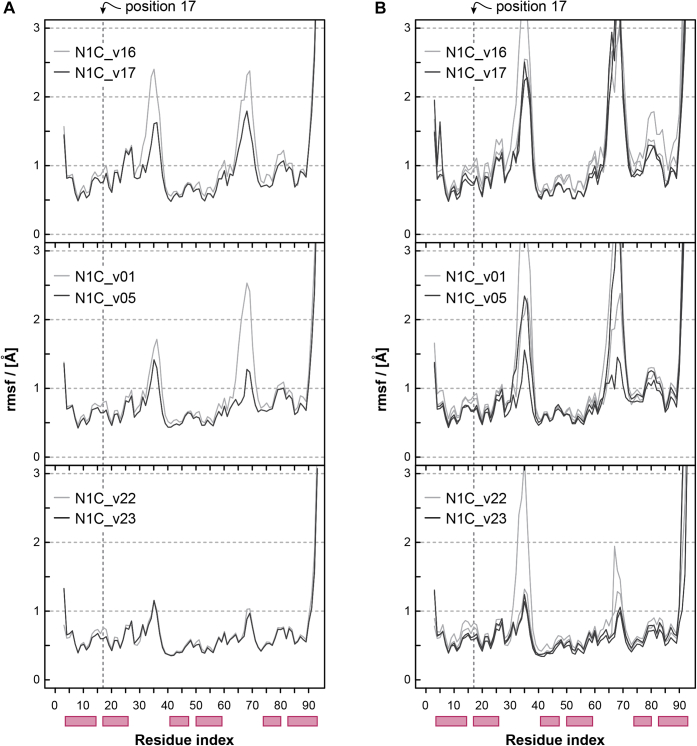
Figure 5.
RMSF profiles of the proteins at 400 K. Compared are the Asp17 (light gray) with the Leu17 (black) constructs in the N01-background (top), the N02-background (middle), and the N02-background combined with the mut5 C-Cap (bottom). Two different timescales are shown comprising (a) fluctuations over 5 ns and (b) 150 ns. The RMSF profiles on the 5 ns timescale were calculated as the average over 30 independent 5-ns profiles. The four independent profiles for the 150 ns timescales show the fluctuations over the first and the last 150 ns of the production simulations that is, the last 300 ns of the 600 ns run. The fluctuations were calculated about the average structure determined from the corresponding 5 ns or 150 ns intervals. The stability of the folded structure of the Leu17-constructs is higher than the Asp17-constructs as the former show lower Cα RMSF. The helical regions of the secondary structure are indicated by red boxes below the residue index.