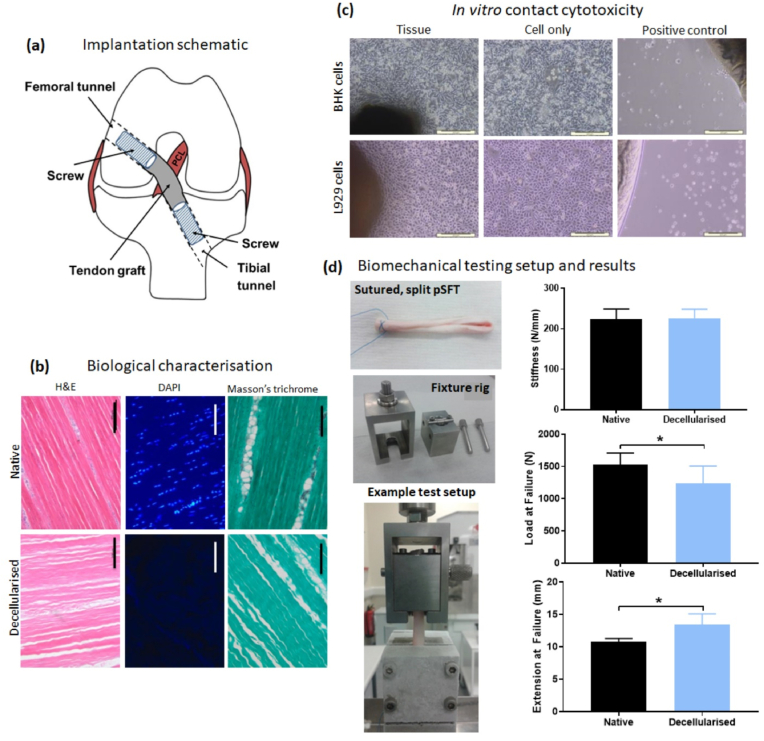
Fig. 1.
Implantation schematic and quality assurance of decellularised pSFT for implantation. A schematic of graft placement is provided for reference (a). Biological characterisation shows representative images (b) following staining of histological sections of native (top row) and decellularised (bottom row) tissues with H&E, DAPI and Masson's trichrome. The same exposure settings were used for DAPI images of fresh and decellularised pSFT. In vitro contact cytotoxicity testing (c) of decellularised pSFT using BHK (top row) and L929 (bottom row) cell lines. Representative images shown of pSFT samples, cell only wells and cyanoacrylate positive controls; scale bars show 200 μm. Biomechanical testing setup (d) demonstrates the sutured, split tendon, bespoke fixture rig and system clamped in place on the testing machine, as well as the stiffness, load at failure and extension at failure of native and decellularised tendons. Graphs show mean ± 95% confidence intervals. * denotes significant difference between groups (p < 0.05, upaired t-test).