Abstract
Rationale
Dietary nitrate and nitrite have a notoriously bad reputation because of their proposed association with disease, in particular cancer. However, more recent lines of research have challenged this dogma suggesting that intake of these anions also possess beneficial effects after in vivo conversion to the vital signaling molecule nitric oxide. Such effects include improvement in cardiovascular, renal and metabolic function, which is partly mediated via reduction of oxidative stress. A recent study even indicates that low dose of dietary nitrite extends life span in fruit flies.
Methods
In this study, 200 middle-aged Wistar rats of both sexes were supplemented with nitrate or placebo in the drinking water throughout their remaining life and we studied longevity, biochemical markers of disease, vascular reactivity along with careful determination of the cause of death.
Results
Dietary nitrate did not affect life span or the age-dependent changes in markers of oxidative stress, kidney and liver function, or lipid profile. Ex vivo examination of vascular function, however, showed improvements in endothelial function in rats treated with nitrate. Neoplasms were not more common in the nitrate group.
Conclusion
We conclude that chronic treatment with dietary nitrate does not affect life span in rats nor does it increase the incidence of cancer. In contrast, vascular function was improved by nitrate, possibly suggesting an increase in health span.
Keywords: Health span, Aging, Inorganic nitrate, Nitrite, Nitric oxide, Long-term supplementation, Cardiovascular, Cancer
1. Introduction
The role of inorganic nitrate and nitrite in human health has since long attracted scientific interest. In the mid 50's, animal experiments revealed that a group of potentially cancerogenic nitrosamines could be generated in the acidic stomach from protonation of nitrite, generating nitrous acid, that could then nitrosate dietary amines [1]. Since then, a great concern in society has led to strict recommendations and regulations to reduce human exposure to these anions via the food and drinking water.
The major sources of nitrate in our diet are vegetables and to some extent also drinking water [2]. The relevance in considering nitrate as a potential carcinogen is related to its conversion to nitrite by oral commensal bacteria in mammals [3]. Although there is little doubt that some nitrosamines have carcinogenic properties, the question of whether nitrate intake is related to cancer in humans remains unclear. Experimental animal studies performed during the last three decades, using long-term dietary treatment with low-to-high doses of inorganic nitrate have not shown convincing evidence that nitrate is carcinogenic [[4], [5], [6]]. National and international authorities regularly survey the scientific field and publish recommendations on the risk related to intake of nitrate. The World Health Organization's International Agency for Research on Cancer, summarizes that there is inadequate evidence in humans for the carcinogenicity of nitrate in food and water [7]. In addition, the European Food Safety Authority concludes in their survey on nitrate in vegetables that exposures to nitrate from vegetables are unlikely to result in appreciable health risks [8].
Since the discovery of nitric oxide (NO) as an endogenous signaling molecule [9,10] and later that inorganic nitrate can be serially reduced to generate NO bioactivity [11,12], a completely different aspect on the biological role of nitrate and nitrite has emerged among preclinical and clinical scientists within the medical field. It is now evident that a nitrate-nitrite-NO pathway exists alongside the more classical l-arginine-NO synthase (NOS) pathway, with beneficial effects on cardiovascular [13], renal [14] and metabolic functions [15].
In animal disease models, treatment with nitrate and nitrite attenuates hypertension, prevents endothelial dysfunction [16] and protects against ischemia-reperfusion injury in several organs such as heart [17], liver [18] and kidney [19,20], by reducing inflammation, modulating mitochondrial function and by dampening oxidative stress [21,22]. Moreover, nitrate improves cardiometabolic function in animal models of ageing-, genetic- or diet-induced metabolic syndrome [15]. In humans, dietary nitrate reduces blood pressure [23], improves endothelial function [24] and enhances aerobic and muscular efficiency during exercise [25]. Taken together, these results implicate that dietary nitrate may improve health span and life expectancy. However, older long-term studies in rodents, performed to investigate the carcinogenic effects of nitrate by using very high doses, showed varying results in generation of tumors and life span [7]. A recent study by our group, using very low doses of nitrite, showed extended lifespan and prevention of age-related locomotor decline in fruit flies [26].
In light of these opposing views on the actions and effects of inorganic nitrate we aimed to investigate if nitrate administration would affect life span in rats, and at the same time monitor any carcinogenic properties of such an intervention. Nitrate was administered in the drinking water from 15 months of age and the animals were monitored throughout their remaining lifespan.
2. Material and methods
2.1. Animal care and experimental procedure
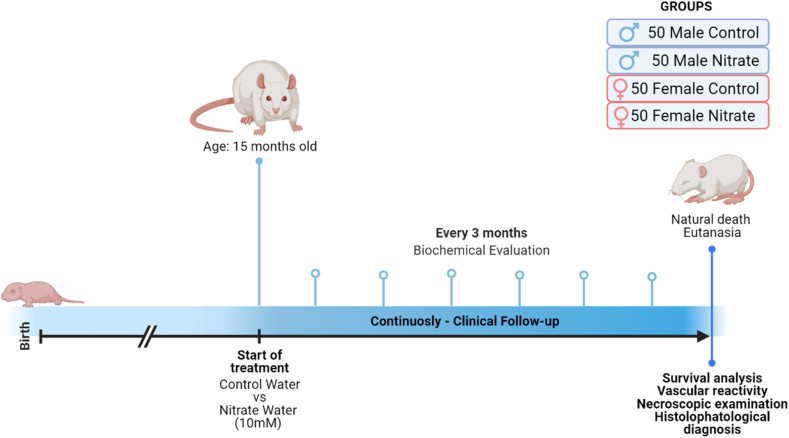
The animal experimental protocol was approved by the Animal Use Ethics Committee of the Federal University of Paraiba – CEUA/UFPB (authorization Nº 1975060318). We used 200 adult Wistar rats (Rattus novergicus), male and female (1:1), aged 15 months, weighing between 250 and 450 g, from the Animal Production Unit - Prof. Thomas George - UFPB. The animals were submitted to medical history evaluations and placed in polysulfone mini-isolators (1154 cm2 floor area), with a maximum capacity of four animals, in a double-face ventilated rack system (40 air changes every 60 min).
The rats were kept under a controlled environment, with constant temperature (22±2 °C) and a 12-h light-dark cycle (i.e. 6am to 6pm; lights on period). The water was filtered and supplied ad libitum through a polysulfone drinker (700 ml), with a stainless-steel spout in a lying model. The Labina Presence® commercial pelleted feed was supplied ad libitum according to the manufacturer's instructions.
This study has a longitudinal prospective character and was designed in parallel in a randomized and placebo-controlled manner. The animals were divided into four groups with 50 rats each: males and females who maintained the supply of filtered water - FEMALE CONTROL (FC) and MALE CONTROL (MC) and rats treated with inorganic nitrate (10 mM) by adding 0.85 g/L of Sodium Nitrate (NaNO3 - Sigma-Aldrich n°221341) to filtered drinking water - FEMALE NITRATE (FN) and MALE NITRATE (MN). The drinking water solutions were changed every 48 h and there were no changes in the feed supply.
The treatment was provided from a pre-established age (15 months) and continued until natural death (Fig. 1). Several studies have demonstrated that supplementation at a dose of 10 mM inorganic nitrate in drinking water is associated with short-term beneficial effects [[27], [28], [29]]. In addition, the positive effect of nitrate consumption on health and diseases progression has already been described by our research group in several other dosages, routes, and experimental models [15].
Fig. 1.
Experimental design. Chronic supplementation with dietary nitrate from 15 months of age, with periodic clinical and biochemical evaluation until natural death or euthanasia and subsequent histopathological diagnosis of the cause of death.
2.2. Experimental follow-up
The animals were followed weekly until death with water consumption, mental state, presence of locomotor disorders, dermatological, dental, and neoplastic alterations being evaluated. Furthermore, the natural death of animals was monitored and recorded (total lifespan).
As this was an observational follow-up study, no concomitant intervention or treatment was performed. However, in cases of detection of any signs of pain and suffering, the animals were promptly euthanized (Humane endpoint), as recommended by the Brazilian National Council of Animal Experimentation Control [30].
2.3. Biochemical assessments
Every three months, plasma samples were collected to assess markers of oxidative stress, kidney and liver function, and lipid profile. For blood collection, animals were contained in acrylic restrainers according to size. Disposable syringes (3 ml) and needles (0.3 × 13 mm) were used for puncture and collection of approximately 1 ml of blood from the lateral tail vein. After collection, the samples were placed in Eppendorf tubes and centrifuged at 3000 rpm for 10 min before freezing.
Plasma oxidative stress was evaluated by analyzing malondialdehyde (MDA), one of the end products of lipid peroxidation, through reactions with thiobarbituric acid (TBARS assay). An aliquot of 250 μl of plasma was separated and submitted to a dry bath at 37 °C for 1 h. Then 400 μl of perchloric acid was added and the mixture centrifuged at 14000 rpm for 20 min at 4 °C. The supernatant was removed, mixed with 400 μl of 0.6% thiobarbituric acid, and incubated at 100 °C for 1 h. After returning to room temperature, the absorbance of the sample was measured at 532 nm in a spectrophotometer and the results were tabulated and expressed as nmol of MDA/ml of plasma.
Plasma concentrations of nitrate+nitrite (NOx) were quantified using a Fluorometric Assay Kit (Cayman Chemical Company® - Ann Arbor, USA). Separate values for nitrate and nitrite could not be achieved so NOx represents the combined levels of nitrate+nitrite.
The other biochemical evaluations in plasma were performed using Bioclin® commercial kits (Minas Gerais, Brazil) and the absorbance was measurement by spectrophotometer (ChemWell-T Automatic Analyzer). Renal function was evaluated by analyzing urea and creatinine levels. Liver function was evaluated by measurement of the extravasation enzymes alanine amino transferase and aspartate amino transferase (ALT-AST); and induction enzymes, alkaline phosphatase and gamma glutamyl transferase (ALP-GGT). The lipid profile was evaluated by measuring total cholesterol and plasma levels of high-density lipoproteins (HDL).
2.4. Vascular reactivity
This study had an end-of-life character, so for the analysis of vascular reactivity, fresh samples from euthanized animals (Humane endpoint) were used. To normalize the evaluation range, only animals that were in dietary supplemental experiment for 12–18 months were used. After euthanasia, a single incision was made in the linea alba to access the abdominal cavity. Subsequently, the cranial mesenteric artery was identified, removed and immediately incubated in Tyrode solution [31].
Then, the artery was cleaned, dissected and sectioned into rings 1–2 mm in length. The rings were immersed in vats (10 ml) and suspended vertically by cotton threads attached to a force transducer (PowerLab™, ADInstruments, MA, USA). The tissues were kept in Tyrode, at 37 °C, gassed with a mixture of 95% O2 and 5% CO2 (carbogen), with a constant pH between 7.2 and 7.4. The rings were then subjected to a basal tension of approximately 0.75 g for a period of 60 min. During this period, the bath solution was changed every 15 min to prevent the interference of metabolites, and the basal tension was adjusted when necessary [32].
Changes in isometric tension were captured by the specific acquisition system (Miobath-4, WPI, Sarasota, USA). After the 60-min stabilization period, the test to assess the endothelial integrity of the vessels was performed. For this, the contraction of the ring was initially induced with phenylephrine (Phe, 10 μM), the rings that obtained a contraction greater than 0.30 g were considered viable. The presence of functional endothelium was verified by the relaxation of the rings after addition of acetylcholine (ACh, 10 μM). Rings with a relaxation greater than 60% over pre-contraction with Phe were considered to have a functional endothelium (E+), a specific value for elderly animals [33]. Furthermore, when the relaxation was less than 60% and greater than 10%, the rings were discarded, and were considered without functional endothelium (E−) when the relaxation was less than 10%.
The contractile response of the rings to Phe was evaluated through the cumulative addition of increasing Phe concentrations (0.1 nM - 10 μM) at 3-min intervals, forming a concentration-response curve of the vasoconstrictor effect. Then, the relaxation of the rings was evaluated, adding cumulative concentrations of vasorelaxant agents at intervals of 3 min between each. For rings with functional endothelium, increasing concentrations of acetylcholine were used (ACh 0.1 nM - 10 μM) and for rings without functional endothelium, concentrations of sodium nitroprusside (SNP 0.1 nM - 10 μM) were used, composing the concentration-response curves. All reagents and salts used in the assessment of vascular reactivity were obtained from Sigma-Aldrich®.
2.5. Histopathological evaluation
In the case of death due to senescence or euthanasia (see section 2.2), necropsy was performed, with macroscopic analysis, photo documentation, and collection of organ samples for histopathological evaluation(s). The samples were immediately stored in a 10% formalin solution for fixation, and later preparation of slides as previously described [34]. The tissues were dehydrated using ethyl alcohol in increasing concentrations, diaffinized in xylene, embedded in liquid paraffin (60 °C), and cut using a microtome (5 μm). Finally, the samples were submitted to hematoxylin-eosin (HE) staining and histopathological analysis by specialized professionals from the Veterinary Pathology Laboratory of the Veterinary Hospital (HV/CCA/UFPB).
2.6. Statistical analysis
The date of death of the animals was used to compose the survival curves (Kaplan-Meier), which were compared by the Log-rank statistical test (Mantel-Cox). The lifespan results and biochemical analyzes were expressed as mean ± standard deviation of the mean. The significance was determined with unpaired t-test for comparisons between two groups, and two-way ANOVA when the additional variable “time” was applicable.
For the concentration-response curves, Emax (Maximum Effect) and pD2 (negative logarithm of the concentration that produces 50% of Emax values were obtained by non-linear regression. A p-value less than 0.05 was considered statistically significant. All statistical analyzes were performed using GraphPad Prism 9.0.
3. Results
3.1. Long term inorganic nitrate supplementation does not affect the longevity
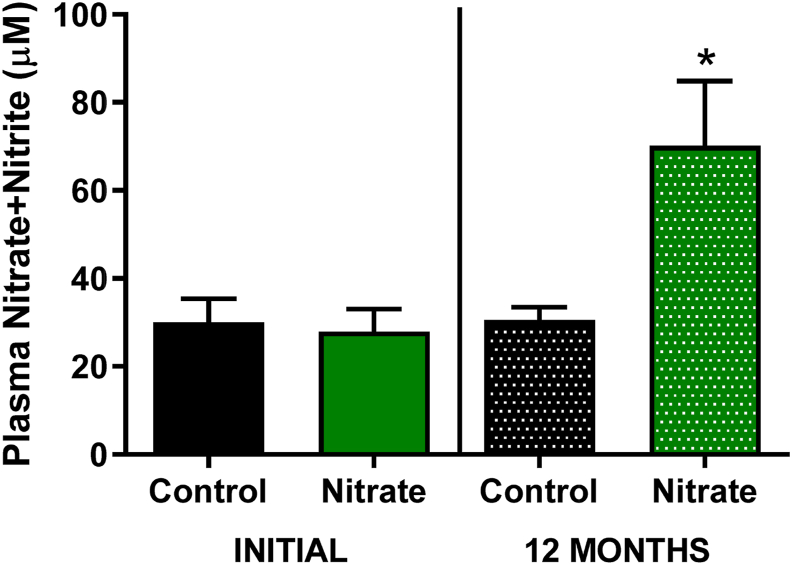
The primary aim of this study was to assess whether chronic consumption of inorganic nitrate affects lifespan. Rats of both sexes were supplemented with nitrate (10 mM) from 15 months of age until death (Mean treatment time = 4066 ± 18.5 days). An increase in plasma concentrations of NOx (nitrate+nitrite) compared to control and baseline values was noted after one year of supplementation (Fig. 2). The interval between birth and death (lifetime) was used to compose the Kaplan-Meier survival curves and statistical comparison with the control groups.
Fig. 2.
Plasma concentrations of NOx (Nitrate+Nitrite) in elderly rats. At 15 months of age (INITIAL) and after 1 year of supplementation with dietary nitrate (12 MONTHS) a significant increase in plasma concentration (*) was identified in both sexes after chronic supplementation with inorganic nitrate NaNO3, 10 mM compared to control and baseline. Values are expressed as mean ± SEM.
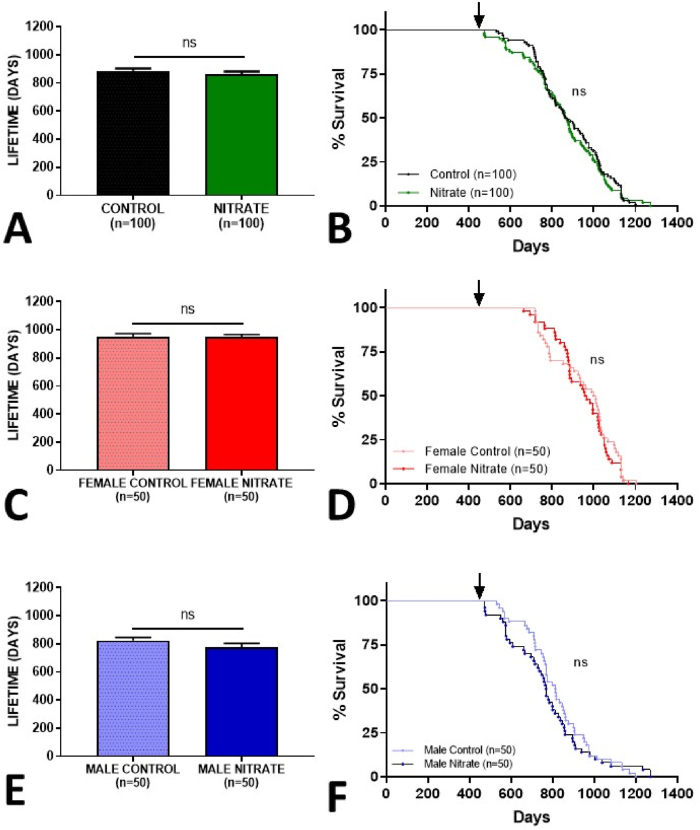
We found that animals chronically supplemented with inorganic nitrate did not show differences in a total lifetime when compared to the control group (p = 0.22) (Fig. 3A). The same was true for the survival analyzes, where there is an overlapping of the Kaplan-Meier survival curves with no difference in the Log-rank test (p = 0.66), and risk of death (Fig. 3B).
Fig. 3.
Lifespan and Kaplan-Meier survival curves of aged animals treated with inorganic nitrate (10 mM NaNO3) from 15 months of age onwards (black arrow). Combined genders (A–B), females (C–D) and males (E–F). Values were considered significant when p<0.05.
Using only data from animals that died naturally (Female: 74%/Male: 70%), no difference was observed (Supplementary Fig. S1). Also, long-term consumption of nitrate was not responsible for differences in the proportion of animals that died naturally or were euthanized (human endpoint).
Our group demonstrated recently in a short-lived invertebrate model (i.e. fruit flies), that the consumption of very low dietary doses of nitrite (0.1 and 1 μM) can increase life-span. However, the results were evident only in females, suggesting a possible sex-specific effect of this anion [26].
To clarify this, in our mammalian longevity experiment, we separated the groups with respect to gender. The group of female rats that consumed nitrate had the same total lifetime (p = 0.94) and no difference between the survival curves (p = 0.46) in females consuming the control solution (Fig. 3C and D).
For males, the results were in the same line, the animals that consumed nitrate had a total lifetime equal to the control group (p = 0.1159), with no changes in the survival analyses (p = 0.55), and risk of death (Fig. 3E and F). As expected, we also demonstrated that males live shorter than females (Supplementary Fig. S1).
In aggregate, our results suggest that long-term consumption of inorganic nitrate is safe, does not increase the risk of death, neither influences the lifetime of animals regardless of gender.
3.2. Long-term inorganic nitrate supplementation does not increase the incidence of cancer and age-related diseases
Supplementation with inorganic nitrate and increased consumption of nitrate-rich vegetables have been associated with salutary effects on cardiovascular, renal and metabolic function [15]. However, throughout history, nitrate has predominantly been related to deleterious health effects, in particular due to possible formation of carcinogenic nitrosamines [35,36].
In order to investigate the relationship of chronic nitrate consumption with the development of spontaneous illnesses, our longevity experiment was designed in an “end of life” model, where animals were followed until the end of life without medical intervention. After natural death or euthanasia (humane endpoint), a complete post-mortem examination with histopathological analysis of the organs was performed (Supplementary Fig. S2).
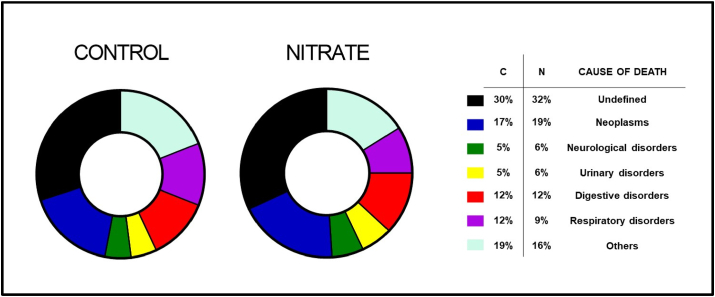
Pathological alterations were organized by the organic system involved, except for neoplasms, composing a separate group. The results are shown in Table 1. Chronic consumption of nitrate was not related to any variation in the incidence of cancer or diseases involved in the cause of death of the animals (Fig. 4).
Table 1.
Description of causes of death, diagnosed by histopathology in male and female rats, submitted to chronic treatment with inorganic nitrate NaNO3 10 mM, from 15 months of age until death. *Humane endpoint.
| CONTROL♀ | NITRATE♀ | CONTROL♂ | NITRATE♂ | |
|---|---|---|---|---|
| Neoplasms | ||||
| Fibroma | 3 | 4 | 1 | 2 |
| Fibrosarcoma | 3 | 3 | 1 | – |
| Cystic leiomyoma | 1 | – | 1 | 1 |
| Breast Fibroadenoma | 2 | 1 | – | – |
| Hemangiosarcoma | 1 | – | 1 | – |
| Pancreatic adenocarcinoma | – | 1 | – | – |
| Myxoma | – | 1 | – | – |
| Lipoma | 2 | 2 | – | 2 |
| Uterine adenocarcinoma | 1 | 1 | – | – |
| Granulosa cell tumor | – | 1 | – | – |
| Gastric cancer | – | – | – | – |
| Neurological system | ||||
| Head tilt* | – | 1 | 2 | 1 |
| Uremic encephalopathy | 1 | 1 | 2 | 3 |
| Urinary system | ||||
| End-stage renal disease | 2 | 1 | 3 | 4 |
| Polycystic kidneys | – | 1 | – | – |
| Digestive system | ||||
| Dental changes* | 1 | 1 | 4 | 3 |
| Hepatic lipidosis | 3 | 3 | 4 | 5 |
| Respiratory system | ||||
| Granulomatous pneumonia | 4 | 2 | 4 | 3 |
| Uremic lung disease | 1 | – | 2 | 2 |
| Pulmonary emphysema | – | 2 | 1 | |
| Others | ||||
| Alopecia/Ulcerative lesions* | 7 | 6 | 5 | 7 |
| Polycystic ovary syndrome | 2 | 1 | – | – |
| Pyometra/Mucometra | 4 | 2 | – | – |
| Endometritis | 1 | – | – | – |
| TOTAL (%) | 39 (78%) | 35 (70%) | 31 (62%) | 33 (66%) |
Fig. 4.
Distribution of the cause of death diagnosed by histopathology in aging rats, treated with inorganic nitrate (NaNO3 10 mM), from 15 months of age until death. (Combined genders, C = Control/N = Nitrate).
However, regardless of nitrate supplementation, females had a greater natural development of neoplasms (relative risk 5.3 x greater), especially tumors originating from connective tissue such as fibroma and fibrosarcoma (Table 1; Supplementary Fig. S3). This would be in accordance with previous reports on gender differences related to the spontaneous development of pathological conditions in laboratory animals [37,38].
Finally, in approximately 31% of the cases it was not possible to diagnose the cause of death (Undefined - Fig. 4), mainly because the animals were found in an advanced state of autolysis making the evaluation impossible, or when death due to senescence occurred without histopathological changes leading to a diagnostic association, such as some cardiovascular diseases.
3.3. Time-dependent organ functional decline is not affected by nitrate consumption
The aging process is characterized by the progressive loss of physiological integrity, leading to impaired function and greater risk of death [39]. In organic systems, in addition to time-dependent structural and functional changes, there is also a greater predisposition to pathological changes, such as the compromised immune system and increased oxidative stress.
Beneficial effects of dietary nitrate have mainly been described following short-term supplementation and in disease models using relatively young animals. Isolated studies demonstrate beneficial effects of nitrate consumption on the lipid profile [40], and liver function [41] of elderly animals. Thus, to investigate the effect of aging and chronic consumption of inorganic nitrate, samples were collected every 3 months for measurement of plasma biomarkers of kidney and liver function, oxidative stress and lipid profile.
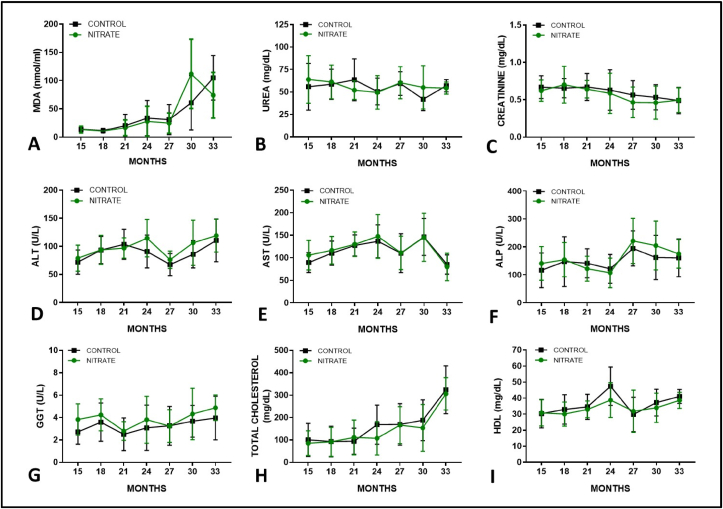
In our experiment, altered characteristics was associated with aging, but without any significant differences between controls and nitrate treated animals (Fig. 5). Oxidative stress was evaluated by quantifying malondealdehyde (MDA), one of the end products of cell lipid peroxidation; and as expected, it was possible to identify a time-dependent, progressive increase in plasma concentrations (Fig. 5A).
Fig. 5.
Biochemical evaluation of plasma markers of oxidative stress (MDA), renal function (Urea - Creatinine), liver injury (ALT -AST), impaired bile flow (ALP - GGT) and lipid profile (Total cholesterol - HDL) in aging rats chronically treated with inorganic nitrate NaNO3, 10 mM. Sample description and gender data in the supplemental content. Values expressed as mean ± SEM.
As for the kidneys, the development of severe functional disorders requires a great loss of viable nephrons. Therefore, due to the reserve capacity of the renal tissue, only mild changes are commonly observed over time [42]. At this point, we demonstrated a slight and progressive reduction in plasma urea and creatinine concentrations (Fig. 5B and C), but no differences between animals that consumed nitrate and control solution.
Finally, enzymes that indicate the presence of direct injury to hepatocytes (Fig. 5D and E), impaired bile flow (Fig. 5F and G) and markers of liver function/lipid profile (Fig. 5H and I) demonstrate the effect of time on liver activity. However, corroborating the previous findings, with no differences in the consumption of nitrate, suggesting that this supplementation is not harmful to organ function in the long term.
The results were arranged according to the intervention. Further, in order to investigate the effect of nitrate consumption on organ functions according to gender, the same indicators were investigated and compared separately between females (Supplementary Fig. S4) and males (Supplementary Fig. S5). Furthermore, due to the “end-of-life” design of the experiment, the number of live animals for evaluation also suffered a progressive reduction (Supplementary Table S1).
3.4. Long term inorganic nitrate supplementation improves vascular reactivity without signs of tolerance
The use of nitroglycerin in the treatment of angina pectoris began after its original synthesis in 1847. Since then, organic nitrates, and more recently inorganic nitrate, have been widely related to beneficial effects on cardiovascular function through NO-dependent and independent mechanisms [43,44].
However, chronic exposure to organic nitrates often leads to tolerance and progressive reduction of its vasodilator effect. This reaction is still poorly understood and constitutes a limiting factor for clinical use [45]. Thereby, inorganic nitrate has gained prominence in cardiovascular therapy over the past 20 years, despite that their long-term effects are still not fully understood.
To investigate this question, we used cranial mesenteric vascular rings from elderly rats in an isolated organ bath system (ex vivo) to evaluate vascular reactivity. The assessment range was normalized (accordingly with standardized protocol [33]) and animals that received nitrate and control solution for at least 1 year and at most 1.5 years (age range 27–33 months) were used.
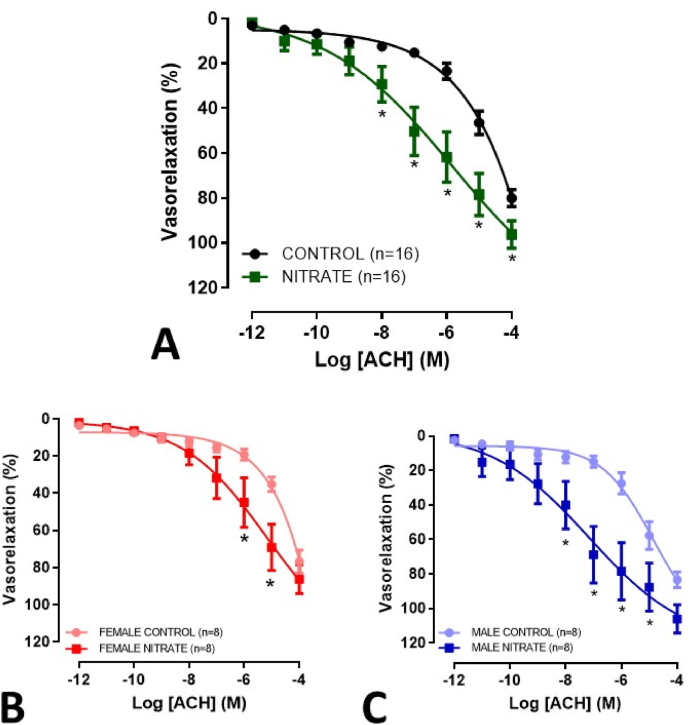
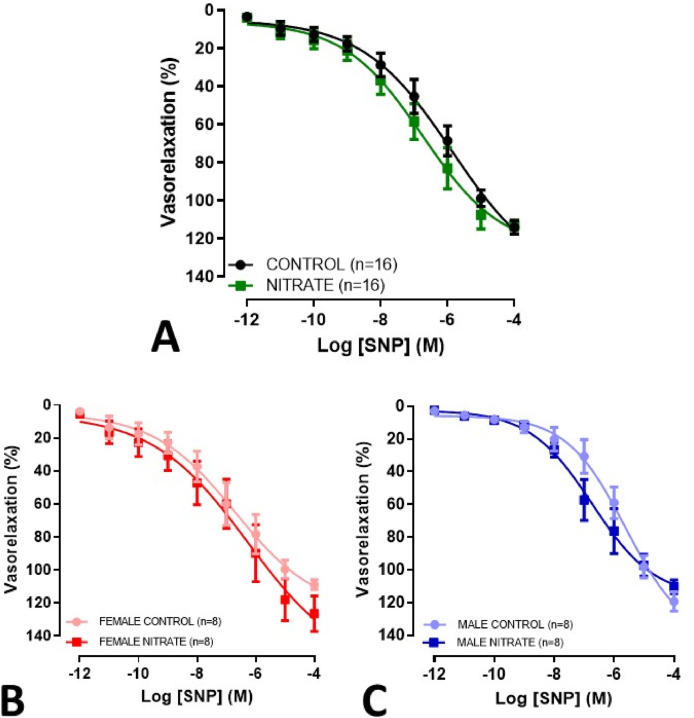
Chronic treatment with nitrate was associated with a considerable improvement in acetylcholine-mediated vasodilation in rings with functional endothelium present, as evident by an increase in maximum response and sensitivity (Fig. 6A, Table 2). These results were apparent in both males and females (Fig. 6B and C). In rings without functional endothelium, no changes were evident regarding the vasodilator effect of sodium nitroprusside (Fig. 7; Table 2). Also, no difference was observed when evaluating the vasoconstrictor effect of Phe in rings with and without functional endothelium (Supplementary Figs. S6–7; Supplementary Table S2).
Fig. 6.
Concentration-response curves for ACH-mediated relaxation (10−12 to 10−4 M), in mesenteric rings with functional endothelium present, from elderly rats treated with inorganic nitrate for more than 12 months. Combined genders (A), females (B) and males (C). *p<0.05.
Table 2.
Values (%) of Emax and pD2 referring to the vasorelaxant effect of acetylcholine (ACH) and sodium nitroprusside (SNP) in rings of the cranial mesenteric artery of elderly animals, of both sexes, chronically treated with inorganic nitrate NaNO3, 10 mM.
| COMBINED GENDERS (n = 16) | FUNCTIONAL ENDOTHELIUM PRESENT (ACH) |
FUNCTIONAL ENDOTHELIUM ABSENT (SNP) |
||
|---|---|---|---|---|
| Emax | pD2 | Emax | pD2 | |
| CONTROL | 80.04 ± 3,69 | 5.38 ± 0.08 | 114.10 ± 3.37 | 6.61 ± 0.12 |
| NITRATE |
96.18 ± 5.96* |
6.98 ± 0.21** |
113.00 ± 2.38 |
6.99 ± 0.15 |
|
GROUPS (n = 8) |
FUNCTIONAL ENDOTHELIUM PRESENT (ACH) |
FUNCTIONAL ENDOTHELIUM ABSENT (SNP) |
||
|
Emax |
pD2 |
Emax |
pD2 |
|
| FEMALE CONTROL | 76.76 ± 6.03 | 5.11 ± 0.09 | 108.85 ± 2.82 | 7.22 ± 0.19 |
| FEMALE NITRATE | 86.26 ± 7,61 | 6.34 ± 0.22* | 115.91 ± 1.88 | 7.11 ± 0.25 |
| MALE CONTROL | 83.33 ± 4,35 | 5.59 ± 0.11 | 119.30 ± 5.75 | 6.10 ± 0.11 |
| MALE NITRATE | 106.10 ± 8.13* | 7.52 ± 0.31** | 110.40 ± 4.06 | 6.89 ± 0.14 |
Values expressed as mean ± SEM. *p<0.05 **p<0.01 Control vs Nitrate.
Fig. 7.
Concentration-response curves for SNP-mediated relaxation (10−12 to 10−4 M), in mesenteric rings lacking functional endothelium, from elderly rats treated with inorganic nitrate for more than 12 months. Combined genders (A), females (B) and males (C). *p<0.05.
Our data suggest that even with uninterrupted consumption for more than 1 year, the improvements in vascular reactivity associated with inorganic nitrate are persistent. These effects were mediated by the endothelium and were similar in both sexes, as described earlier in short-term studies [[46], [47], [48]].
4. Discussion
Over the past decades there has been a great debate regarding the potential carcinogenic effect of inorganic nitrate, which is predominantly found in vegetables and to a minor extent also in our drinking water. At the same time, short term supplementation with this anion has been shown to restore NO bioactivity in experimental disease models of cardiovascular, renal and metabolic disorders. Here we extensively examined the health effects of dietary inorganic nitrate in rats with the intervention starting at midlife and lasting throughout the remaining lifespan. We find that nitrate does not affect longevity, and importantly show that it is not associated with increased incidence of cancer. Nor did supplementation with nitrate significantly alter age-dependent markers of oxidative stress, kidney and liver function, or lipid profile. Nitrate, however, did seem to partly prevent an aging-dependent decline in cardiovascular function, measured as an improvement in endothelial function in ex vivo vessel preparation.
When designing the current study, we aimed at following the guidelines for large-scale comprehensive evaluation methods outlined in the US National Toxicology Program (NTP) cancer bioassay protocols [[49], [50], [51]]. The NTP protocol suggests 2-year exposure periods, a broad range of tissues histologically examined in addition to mortality and body mass measures, the use of a large number of female and male animals (usually 50 animals per gender and group) in addition to the concurrent controls in the individual study [52,53]. Here we used a single rather high dose of nitrate and treated the rats from around midlife. Obviously, the use of multiple doses would have been even more preferable, but it is difficult to say if a higher or lower dose would have given different results. Carlström and colleagues described, in relatively young animals, a crosstalk between the nitrate-nitrite-NO pathway and vascular eNOS in rats, with eNOS activity being down-regulated by the very same dose of nitrate (10 mM) [54]. It is therefore possible, that some positive eNOS-mediated health effects, for example on metabolic function15, might have been counteracted by the high nitrate dose used in the current study. Speaking against this notion, however, is the preserved or even better endothelial (eNOS) function in the nitrate treated group compared with controls. These results are actually in line with previous finding by Hezel et al., showing that two-weeks treatment with a similar dose of nitrate in elderly rats improved endothelial relaxation to acetylcholine and attenuated contractility to angiotensin II [55].
Moretti and colleagues recently showed a surprising increase in longevity in fruit flies after dietary nitrite, possibly implying that inorganic nitrate would actually extend lifespan [26]. Of course, it is difficult to compare different species, and more importantly, the dose of nitrite used in the cited study was minute (0.1–1 μM in the fly food). The dose of nitrate used in the current rat study (approx. 50 mg/kg/day) is considerably higher than would be achievable in humans through a normal diet [2] and well above the Acceptable Daily Intake (ADI) for nitrate which is set to 3.7 mg/kg/day.
One should keep in mind that humans and rodents differ greatly in their overall metabolism of nitrate, so any results looking at long-term effects in rats must be interpreted with caution. As an example, humans actively concentrate nitrate in saliva [56,57] which is not the case in rodents [58]. In saliva nitrate is readily converted to nitrite by oral commensal bacteria. This means that humans are generally more exposed to nitrite at any given intake of nitrate. On the other hand, rodents have a greater NOS activity [59] which contributes to higher endogenous levels of nitrate and nitrite. Moreover, gastric pH is generally lower in humans, particularly under fasting conditions [60], which likely results in more pronounced generation of reactive nitrogen oxides from nitrite in saliva [61]. Also, rodents synthesize ascorbic acid endogenously and excrete it in the gastrointestinal tract, while humans rely on dietary intake of this vitamin [62]. Ascorbic acid acts as an inhibitor of nitrosation reactions and the high content of this vitamin in vegetables is suggested to explain why this food group is not associated with cancer in humans despite being the major source of dietary nitrate [63]. One limitation of the present study is the fact that we were unable to measure nitrate and nitrite separately so what we present (nitrate+nitrite) really is merely a reflection of nitrate exposure, keeping in mind that this anion dominates greatly over nitrite in plasma and tissues. Also, measurements of N-nitrosamines would have been informative. Having said this, one should be aware that with this dose of nitrate and when administering it orally over a long period of time, measurable increases in systemic levels of nitrite are commonly modest or not even seen [55].
The fact that endothelial function was better preserved in nitrate-treated rats is interesting although this apparently did not affect overall lifespan. These results are well in line with previous results obtained in rodents and humans [64,65] and indicate that health span might have improved. The mechanism behind preservation of endothelial function can only be speculated upon at this stage. It may be related to an increase in the expression and/or activity of eNOS as suggested [66] or alternatively it may be related to preserved NO bioavailability in the vascular wall e.g through nitrate-dependent inhibition of vascular NADPH-oxidases [27,55], thereby lowering the levels of superoxide which would rapidly scavenge NO. In any case, although we did not measure endothelial function before the intervention, these results also indicate that long-term treatment with inorganic nitrate is not associated with development of tolerance, as is the case with traditional organic nitrates.
Dietary nitrate has been associated with negative health effects ever since the discovery of endogenously formed potentially carcinogenic N-nitrosamines, generated through bacteria-dependent nitrate metabolism [35,67,68]. The more recent discovery of NO generation from dietary nitrate with positive effects on cardiovascular, renal and metabolic function in rodents and humans13,14 prompted us to perform a long-term study using a rather high dose of nitrate and looking at neoplasm development, longevity along with assessment of cardio-reno-metabolic functions as well as biochemical analyses of aging-associated changes in markers of oxidative stress, inflammation and lipid homeostasis. We conclude that dietary nitrate has no effect on tumor development or lifespan in rats when treated from midlife. In contrast, vascular function was improved by nitrate, possibly suggesting an increase in health span.
Declaration of competing interest
Jon Lundberg and Eddie Weitzberg are named inventors on patents and patent applications relating to the medical uses of inorganic nitrate and nitrite. The rest of the authors declare no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2021.102209.
Contributor Information
Lucas Rannier R.A. Carvalho, Email: lucas.carvalho@ki.se.
Valdir de Andrade Braga, Email: valdir@cbiotec.ufpb.br.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Magee P.N., Barnes J.M. The production of malignant primary hepatic tumours in the rat by feeding dimethylnitrosamine. Br. J. Cancer. 1956;10:114–122. doi: 10.1038/bjc.1956.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weitzberg E., Lundberg J.O. Novel aspects of dietary nitrate and human health. Annu. Rev. Nutr. 2013;33:129–159. doi: 10.1146/annurev-nutr-071812-161159. [DOI] [PubMed] [Google Scholar]
- 3.Tannenbaum S.R., Sinskey A.J., Weisman M., Bishop W. Nitrite in human saliva. Its possible relationship to nitrosamine formation. J. Natl. Cancer Inst. 1974;53:79–84. [PubMed] [Google Scholar]
- 4.Greenblatt M., Mirvish S.S. Dose-response studies with concurrent administration of piperazine and sodium nitrite to strain A mice. J. Natl. Cancer Inst. 1973;50:119–124. doi: 10.1093/jnci/50.1.119. [DOI] [PubMed] [Google Scholar]
- 5.Maekawa A., et al. Carcinogenicity studies of sodium nitrite and sodium nitrate in F-344 rats. Food Chem. Toxicol. 1982;20:25–33. doi: 10.1016/s0278-6915(82)80005-7. [DOI] [PubMed] [Google Scholar]
- 6.Bryan N.S., Alexander D.D., Coughlin J.R., Milkowski A.L., Boffetta P. Ingested nitrate and nitrite and stomach cancer risk: an updated review. Food Chem. Toxicol. 2012;50:3646–3665. doi: 10.1016/j.fct.2012.07.062. [DOI] [PubMed] [Google Scholar]
- 7.Group I.W. IARC monographs on the evaluation of carcinogenic risks to humans. Ingested nitrate and nitrite, and cyanobacterial peptide toxins. IARC Monogr. Eval. Carcinog. Risks Hum. 2010;94(v-vii):1–412. [PMC free article] [PubMed] [Google Scholar]
- 8.Authority E.F.S. Nitrate in vegetables: scientific opinion of the panel on contaminants in the food chain. EFSA J. 2008;689:1–79. doi: 10.2903/j.efsa.2008.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furchgott R.F., Zawadzki J.V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 10.Ignarro L.J., Buga G.M., Wood K.S., Byrns R.E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. U. S. A. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lundberg J.O., Weitzberg E., Lundberg J.M., Alving K. Intragastric nitric oxide production in humans: measurements in expelled air. Gut. 1994;35:1543–1546. doi: 10.1136/gut.35.11.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benjamin N., et al. Stomach NO synthesis. Nature. 1994;368:502. doi: 10.1038/368502a0. [DOI] [PubMed] [Google Scholar]
- 13.Lundberg J.O., Weitzberg E., Gladwin M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 14.Carlstrom, M. Nitric oxide signalling in kidney regulation and cardiometabolic health. Nat. Rev. Nephrol. 17, 575-590, doi:10.1038/s41581-021-00429-z(2021). [DOI] [PMC free article] [PubMed]
- 15.Lundberg J.O., Carlstrom M., Weitzberg E. Metabolic effects of dietary nitrate in health and disease. Cell Metabol. 2018;28:9–22. doi: 10.1016/j.cmet.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Carlstrom M., Lundberg J.O., Weitzberg E. Mechanisms underlying blood pressure reduction by dietary inorganic nitrate. Acta Physiol. 2018;224 doi: 10.1111/apha.13080. [DOI] [PubMed] [Google Scholar]
- 17.Webb A., et al. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc. Natl. Acad. Sci. U. S. A. 2004;101:13683–13688. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duranski M.R., et al. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J. Clin. Invest. 2005;115:1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang T., et al. Dietary nitrate attenuates renal ischemia-reperfusion injuries by modulation of immune responses and reduction of oxidative stress. Redox Biol. 2017;13:320–330. doi: 10.1016/j.redox.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang G., et al. Renovascular effects of inorganic nitrate following ischemia-reperfusion of the kidney. Redox Biol. 2021;39 doi: 10.1016/j.redox.2020.101836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raubenheimer K., et al. Effects of dietary nitrate on inflammation and immune function, and implications for cardiovascular health. Nutr. Rev. 2019 doi: 10.1093/nutrit/nuz025. [DOI] [PubMed] [Google Scholar]
- 22.Schiffer T.A., Lundberg J.O., Weitzberg E., Carlstrom M. Modulation of mitochondria and NADPH oxidase function by the nitrate-nitrite-NO pathway in metabolic disease with focus on type 2 diabetes. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2020;1866:165811. doi: 10.1016/j.bbadis.2020.165811. [DOI] [PubMed] [Google Scholar]
- 23.Larsen F.J., Ekblom B., Sahlin K., Lundberg J.O., Weitzberg E. Effects of dietary nitrate on blood pressure in healthy volunteers. N. Engl. J. Med. 2006;355:2792–2793. doi: 10.1056/NEJMc062800. [DOI] [PubMed] [Google Scholar]
- 24.Lara J., et al. Effects of inorganic nitrate and beetroot supplementation on endothelial function: a systematic review and meta-analysis. Eur. J. Nutr. 2016;55:451–459. doi: 10.1007/s00394-015-0872-7. [DOI] [PubMed] [Google Scholar]
- 25.Larsen F.J., et al. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metabol. 2011;13:149–159. doi: 10.1016/j.cmet.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Moretti C.H., et al. Dietary nitrite extends lifespan and prevents age-related locomotor decline in the fruit fly. Free Radic. Biol. Med. 2020;160:860–870. doi: 10.1016/j.freeradbiomed.2020.09.018. [DOI] [PubMed] [Google Scholar]
- 27.Gao X., et al. NADPH oxidase in the renal microvasculature is a primary target for blood pressure-lowering effects by inorganic nitrate and nitrite. Hypertension. 2015;65:161–170. doi: 10.1161/HYPERTENSIONAHA.114.04222. [DOI] [PubMed] [Google Scholar]
- 28.Cordero-Herrera I., et al. AMP-activated protein kinase activation and NADPH oxidase inhibition by inorganic nitrate and nitrite prevent liver steatosis. Proc. Natl. Acad. Sci. U. S. A. 2019;116:217–226. doi: 10.1073/pnas.1809406115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guimaraes D.D., et al. Dietary nitrate reduces blood pressure in rats with angiotensin II-induced hypertension via mechanisms that involve reduction of sympathetic hyperactivity. Hypertension. 2019;73:839–848. doi: 10.1161/HYPERTENSIONAHA.118.12425. [DOI] [PubMed] [Google Scholar]
- 30.Experimentation Guidelines for the practice of euthanasia of CONCEA. Resolution. 2019;45:54. C.-B. N. C. f. t. C. o. A. [Google Scholar]
- 31.Tanaka Y., Mochizuki Y., Tanaka H., Shigenobu K. Significant role of neuronal non-N-type calcium channels in the sympathetic neurogenic contraction of rat mesenteric artery. Br. J. Pharmacol. 1999;128:1602–1608. doi: 10.1038/sj.bjp.0702954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Altura B.M., Altura B.T. Differential effects of substrate depletion on drug-induced contractions of rabbit aorta. Am. J. Physiol. 1970;219:1698–1705. doi: 10.1152/ajplegacy.1970.219.6.1698. [DOI] [PubMed] [Google Scholar]
- 33.Matz R.L., de Sotomayor M.A., Schott C., Stoclet J.C., Andriantsitohaina R. Vascular bed heterogeneity in age-related endothelial dysfunction with respect to NO and eicosanoids. Br. J. Pharmacol. 2000;131:303–311. doi: 10.1038/sj.bjp.0703568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carvalho L.R.R.A.P., Pereira H.C.D.S., Silva H.T.F., Lucena R.B., Guerra R.R. Creolin® administered by different pathways in rats experimentally poisoned with Bothrops jararaca venom. Ciência Rural. 2019;49:1–7. [Google Scholar]
- 35.Tannenbaum S.R., Correa P. Nitrate and gastric cancer risks. Nature. 1985;317:675–676. doi: 10.1038/317675b0. [DOI] [PubMed] [Google Scholar]
- 36.Song P., Wu L., Guan W. Dietary nitrates, nitrites, and nitrosamines intake and the risk of gastric cancer: a meta-analysis. Nutrients. 2015;7:9872–9895. doi: 10.3390/nu7125505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carlus M., Elies L., Fouque M.C., Maliver P., Schorsch F. Historical control data of neoplastic lesions in the Wistar Hannover Rat among eight 2-year carcinogenicity studies. Exp. Toxicol. Pathol. 2013;65:243–253. doi: 10.1016/j.etp.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 38.Snyder J.M., Ward J.M., Treuting P.M. Cause-of-Death analysis in rodent aging studies. Vet. Pathol. 2016;53:233–243. doi: 10.1177/0300985815610391. [DOI] [PubMed] [Google Scholar]
- 39.Lopez-Otin C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li T., Lu X., Sun Y., Yang X. Effects of spinach nitrate on insulin resistance, endothelial dysfunction markers and inflammation in mice with high-fat and high-fructose consumption. Food Nutr. Res. 2016;60:32010. doi: 10.3402/fnr.v60.32010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang H., et al. Inorganic nitrate alleviates the senescence-related decline in liver function. Sci. China Life Sci. 2018;61:24–34. doi: 10.1007/s11427-017-9207-x. [DOI] [PubMed] [Google Scholar]
- 42.Meuten D. In: Guanabara Koogan. Thrall M.A., editor. 2017. In hematology and clinical veterinary biochemistry. [Google Scholar]
- 43.Kapil V., Khambata R.S., Robertson A., Caulfield M.J., Ahluwalia A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension. 2015;65:320–327. doi: 10.1161/HYPERTENSIONAHA.114.04675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Munzel T., Daiber A. Inorganic nitrite and nitrate in cardiovascular therapy: a better alternative to organic nitrates as nitric oxide donors? Vasc. Pharmacol. 2018;102:1–10. doi: 10.1016/j.vph.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Divakaran S., Loscalzo J. The role of nitroglycerin and other nitrogen oxides in cardiovascular therapeutics. J. Am. Coll. Cardiol. 2017;70:2393–2410. doi: 10.1016/j.jacc.2017.09.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bakker J.R., et al. Low dose dietary nitrate improves endothelial dysfunction and plaque stability in the ApoE(-/-) mouse fed a high fat diet. Free Radic. Biol. Med. 2016;99:189–198. doi: 10.1016/j.freeradbiomed.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 47.Peng R., Luo M., Tian R., Lu N. Dietary nitrate attenuated endothelial dysfunction and atherosclerosis in apolipoprotein E knockout mice fed a high-fat diet: a critical role for NADPH oxidase. Arch. Biochem. Biophys. 2020;689:108453. doi: 10.1016/j.abb.2020.108453. [DOI] [PubMed] [Google Scholar]
- 48.Rammos C., et al. Dietary nitrate is a modifier of vascular gene expression in old male mice. Oxid. Med. Cell. Longev. 2015 doi: 10.1155/2015/658264(2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beyer L.A., Beck B.D., Lewandowski T.A. Historical perspective on the use of animal bioassays to predict carcinogenicity: evolution in design and recognition of utility. Crit. Rev. Toxicol. 2011;41:321–338. doi: 10.3109/10408444.2010.541222. [DOI] [PubMed] [Google Scholar]
- 50.Pastoor, T. & Stevens, J. Historical perspective of the cancer bioassay. Scand. J. Work. Environ. Health 31 Suppl 1, 129-140; discussion 119-122 (2005). [PubMed]
- 51.Program N.T. National Toxicology Program; 2011. Descriptions of NTP Study Types: Toxicology/Carcinogenicity. [Google Scholar]
- 52.Program N.T. National Toxicology Program) 2011. [Google Scholar]
- 53.Rhomberg L.R., et al. Issues in the design and interpretation of chronic toxicity and carcinogenicity studies in rodents: approaches to dose selection. Crit. Rev. Toxicol. 2007;37:729–837. doi: 10.1080/10408440701524949. [DOI] [PubMed] [Google Scholar]
- 54.Carlstrom M., et al. Cross-talk between nitrate-nitrite-NO and NO synthase pathways in control of vascular NO homeostasis. Antioxidants Redox Signal. 2015;23:295–306. doi: 10.1089/ars.2013.5481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hezel M., et al. Dietary nitrate improves age-related hypertension and metabolic abnormalities in rats via modulation of angiotensin II receptor signaling and inhibition of superoxide generation. Free Radic. Biol. Med. 2016;99:87–98. doi: 10.1016/j.freeradbiomed.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 56.Lundberg J.O., Govoni M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic. Biol. Med. 2004;37:395–400. doi: 10.1016/j.freeradbiomed.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 57.Spiegelhalder B., Eisenbrand G., Preussmann R. Influence of dietary nitrate on nitrite content of human saliva: possible relevance to in vivo formation of N-nitroso compounds. Food Chem. Toxicol. 1976;14:545–548. doi: 10.1016/s0015-6264(76)80005-3. [DOI] [PubMed] [Google Scholar]
- 58.Montenegro M.F., et al. Profound differences between humans and rodents in the ability to concentrate salivary nitrate: implications for translational research. Redox Biol. 2016;10:206–210. doi: 10.1016/j.redox.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wickman A., et al. A technique to estimate the rate of whole body nitric oxide formation in conscious mice. Nitric Oxide. 2003;9:77–85. doi: 10.1016/j.niox.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 60.Fujimori S. Gastric acid level of humans must decrease in the future. World J. Gastroenterol. 2020;26:6706–6709. doi: 10.3748/wjg.v26.i43.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lundberg J.O., Weitzberg E. Biology of nitrogen oxides in the gastrointestinal tract. Gut. 2013;62:616–629. doi: 10.1136/gutjnl-2011-301649. [DOI] [PubMed] [Google Scholar]
- 62.Rooke J.A., Skinner E.R. The dissociation of apolipoproteins from rat plasma lipoproteins during isolation by precipitation with polyanions. Int. J. Biochem. 1979;10:329–335. doi: 10.1016/0020-711x(79)90098-3. [DOI] [PubMed] [Google Scholar]
- 63.Mirvish S.S. Experimental evidence for inhibition of N-nitroso compound formation as a factor in the negative correlation between vitamin C consumption and the incidence of certain cancers. Cancer Res. 1994;54:1948s–1951s. [PubMed] [Google Scholar]
- 64.Carlstrom M., Moretti C.H., Weitzberg E., Lundberg J.O. Microbiota, diet and the generation of reactive nitrogen compounds. Free Radic. Biol. Med. 2020;161:321–325. doi: 10.1016/j.freeradbiomed.2020.10.025. [DOI] [PubMed] [Google Scholar]
- 65.Lundberg J.O., Gladwin M.T., Weitzberg E. Strategies to increase nitric oxide signalling in cardiovascular disease. Nat. Rev. Drug Discov. 2015;14:623–641. doi: 10.1038/nrd4623. [DOI] [PubMed] [Google Scholar]
- 66.Ling W.C., Lau Y.S., Murugan D.D., Vanhoutte P.M., Mustafa M.R. Sodium nitrite causes relaxation of the isolated rat aorta: by stimulating both endothelial NO synthase and activating soluble guanylyl cyclase in vascular smooth muscle. Vasc. Pharmacol. 2015;74:87–92. doi: 10.1016/j.vph.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 67.Bartsch H., Ohshima H., Pignatelli B., Calmels S. Endogenously formed N-nitroso compounds and nitrosating agents in human cancer etiology. Pharmacogenetics. 1992;2:272–277. doi: 10.1097/00008571-199212000-00005. [DOI] [PubMed] [Google Scholar]
- 68.Tannenbaum S.R., Young V.R. Endogenous nitrite formation in man. J. Environ. Pathol. Toxicol. 1980;3:357–368. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.