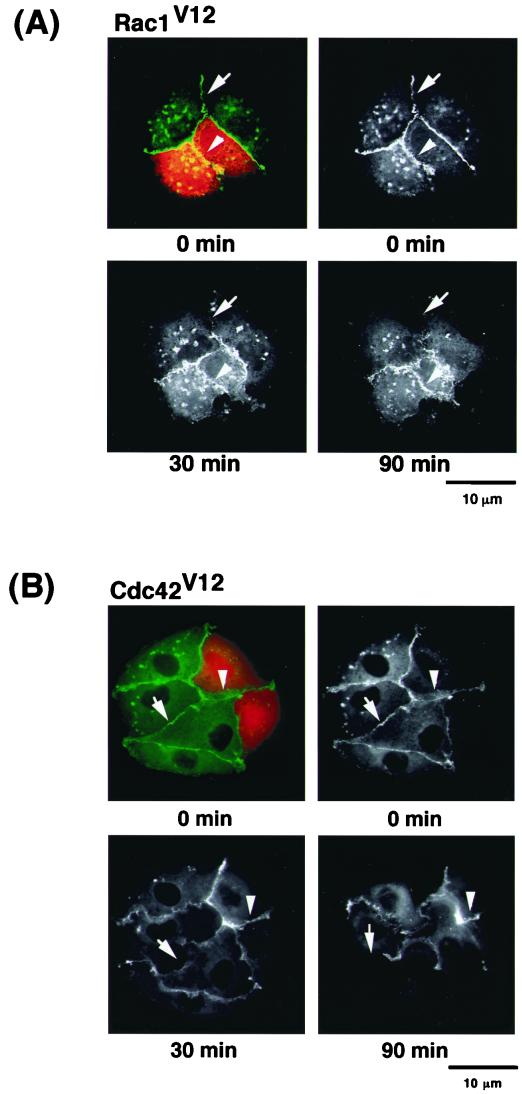
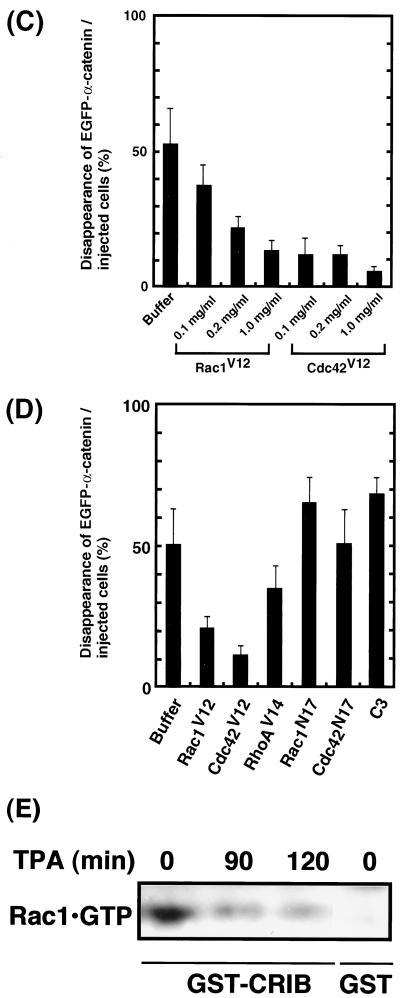
FIG. 5.
Effects of small GTPases on TPA-induced disappearance of EGFP–α-catenin from sites of cell-cell contact. (A) Inhibition of TPA-induced disappearance of EGFP–α-catenin and cell-cell dissociation by constitutively active Rac1 (Rac1V12). Stimulation of the cells with TPA induced the disappearance of EGFP–α-catenin from sites of cell-cell contact before cell-cell dissociation during cell scattering (arrow). In contrast, microinjection of Rac1V12 (0.2 mg/ml) with rhodamine-labeled dextran (red) inhibited both disappearance of EGFP–α-catenin from sites of cell-cell contact and cell-cell dissociation (arrowhead). The time after stimulation is indicated. (B) Similar results were obtained by using constitutively active Cdc42 (Cdc42V12; 0.2 mg/ml) instead of Rac1V12. (C) Rac1V12 and Cdc42V12 inhibited TPA-induced disappearance of EGFP–α-catenin in a dose-dependent manner. Indicated concentrations of Rac1V12 and Cdc42V12 were microinjected as done for panels A and B. At 30 min after the microinjection of the indicated protein, the cells were stimulated with TPA (200 nM) and incubated for 90 min. Data are means ± standard errors of the means of triplicate determinations. (D) Effects of Rac1, Cdc42, and RhoA mutants on TPA-induced disappearance of EGFP–α-catenin. At 30 min after the microinjection of the indicated protein (small GTPase, 0.2 mg/ml each; C3, 0.1 mg/ml), the cells were stimulated with TPA (200 nM) and incubated for 90 min. Data are means ± standard errors of the means of triplicate determinations. (E) MDCKII cells were incubated with TPA (200 nM) for the indicated times. The cells were lysed, and the lysates were incubated with GST-CRIB. The proteins bound to GST-CRIB were subjected to SDS-PAGE, followed by immunoblotting with anti-Rac1 antibody. These results are representative of five independent experiments.