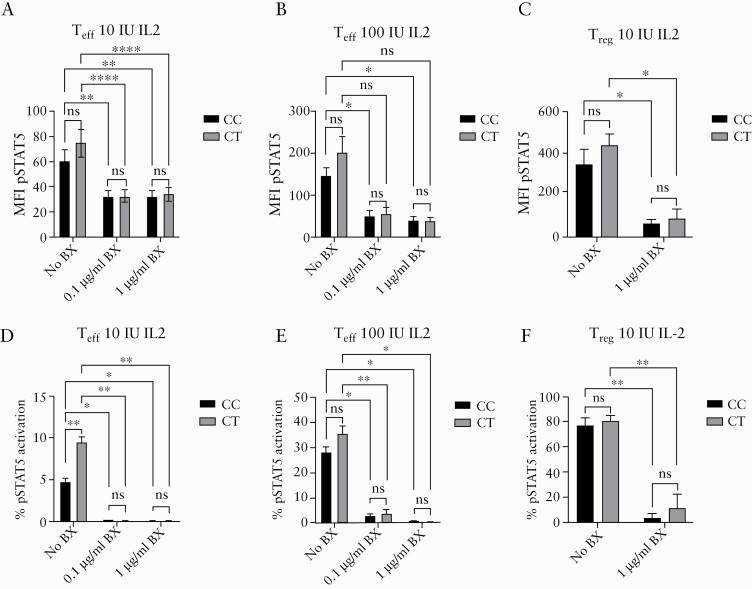
Figure 4.
Basiliximab is effective at blocking phosphorylation of STAT5 in response to IL-2 signalling in rs61839660 heterozygote CD4+ T cells. Basiliximab was added to in vitro cultures of CD4+ effector T cells [Teff] and regulatory T cells [Treg] from heterozygous [CT] and major allele homozygous [CC] Crohn’s disease [CD] patients [n = 3 of each genotype], at the specified concentrations. After 6 h, cells were stimulated with 10 or 100 IU/ml of recombinant human IL-2 for 15 min. [a] Basiliximab was effective in reducing signal transducer and activator of transcription 5 [STAT5] phosphorylation [pSTAT5], quantified as a decrease in the mean fluorescence intensity [MFI] of pSTAT5 flow cytometry staining in Teff stimulated with [a] 10 IU/ml IL-2 or [b] with 100 IU/ml of IL-2. There were no significant differences in basiliximab response between the genotype groups. [c] Basiliximab reduced the MFI for pSTAT5 staining in Treg stimulated with 10 IU of IL-2 in CT and CC subjects. [d] Proportion [%] of pSTAT5 stained Teff activated with 10 IU/ml and [e] 100 IU/ml IL-2 following basiliximab treatment. [f] Proportion [%] of pSTAT5 stained Treg after stimulation with 10 IU/ml IL-2 following basiliximab treatment. Mean ± SEM plotted. Statistical analyses performed using two-way ANOVA, Tukey’s multiple comparisons test for comparisons involving more than two groups, and paired t tests for comparisons involving two treatments within the same genotype. *p <0.05, **p <0.01, ****p <0.0001. BX, basiliximab; Ns, not significant; SEM, standard error of the mean; ANOVA, analysis of variance.