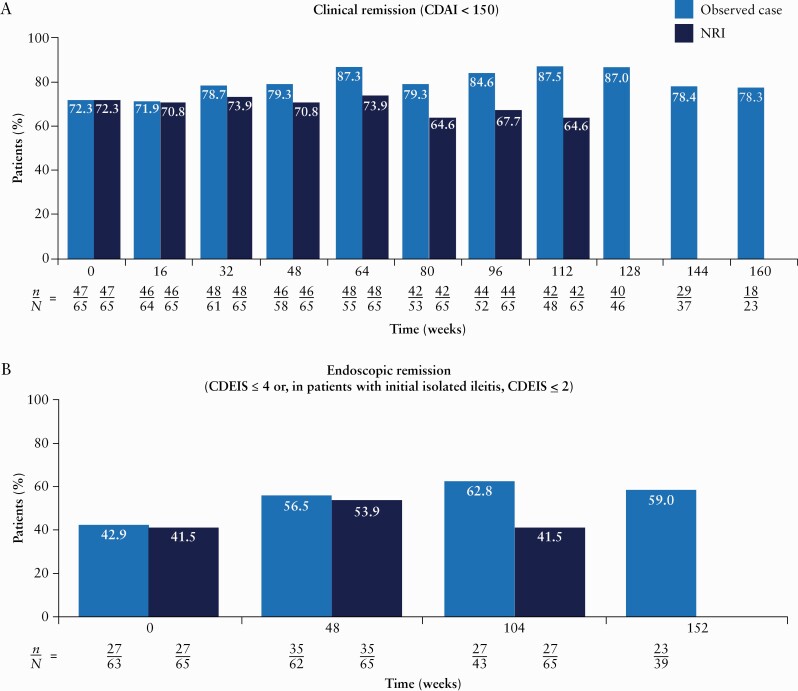
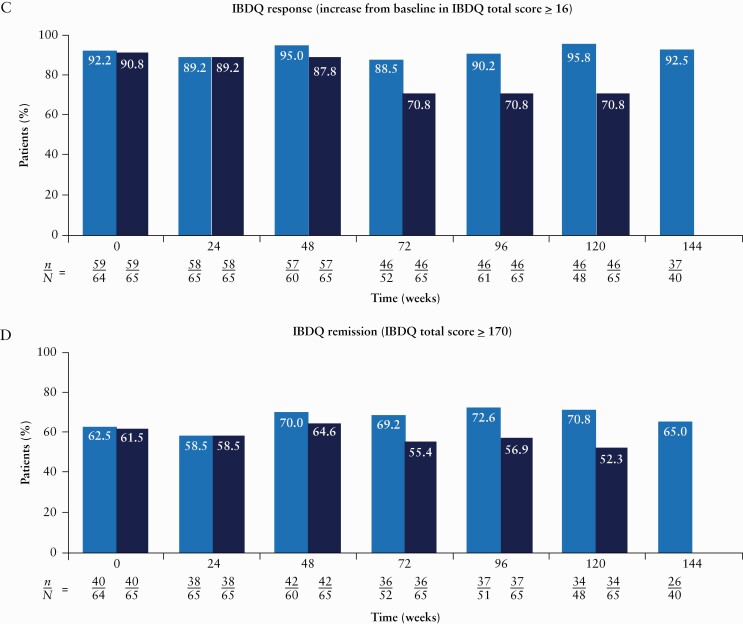
Figure 3.
Proportions of patients achieving clinical remission [A], CDEIS remission [B], IBDQ response [C] and IBDQ remission [D] over time [intent-to-treat analysis set]. From Week 128 onwards, patients could roll over to the ongoing phase 3 OLE [M16-000 sub-study 3]. This resulted in a decrease in the number of patients continuing towards completion in the current phase 2 OLE study and prior to rollover into the phase 3 OLE for further risankizumab treatment and follow-up. Therefore, NRI for missing data was not presented after Week 128. Only observed cases were presented beyond Week 128. n denotes the number of responders and N denotes the number of patients at risk. CDAI, Crohn’s Disease Activity Index; CDEIS, Crohn’s Disease Endoscopic Index of Severity; IBDQ, Inflammatory Bowel Disease Questionnaire; NRI, non-responder imputation; OLE, open-label extension.