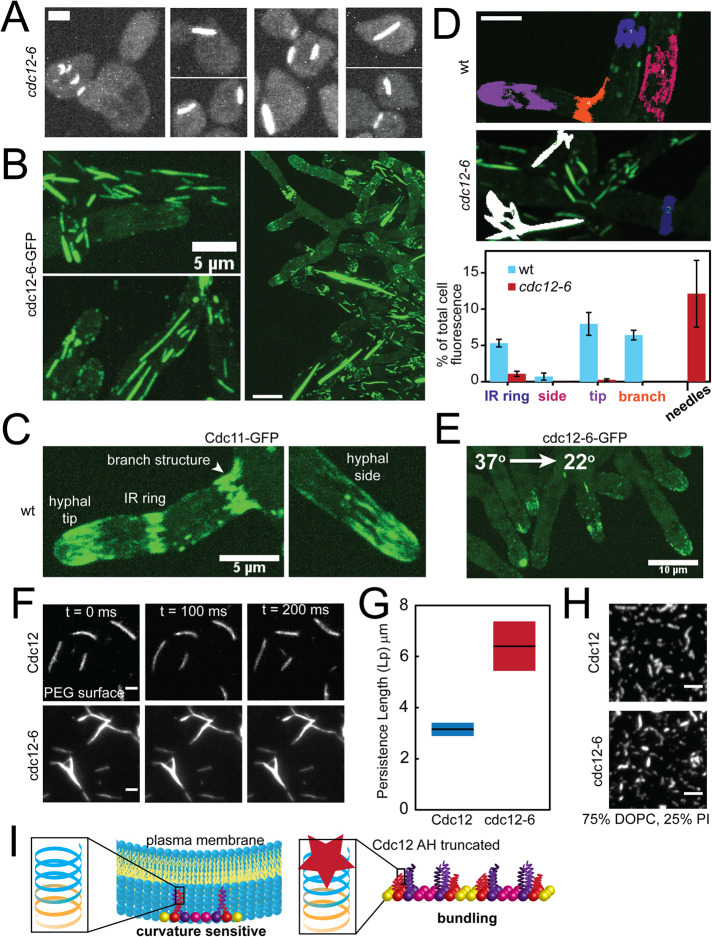
FIGURE 4:
The Cdc12 AH domain inhibits septin bundling. (A) Maximum projections (contrasted identically) of fixed cells with bundled “needles” in cdc12-6 S. cerevisiae mutants expressing Cdc3-mCherry treated with pheromone. Scale bar, 2 µm. (B) Maximum projections of bundled “needles” in A. gossypii expressing cdc12-6-GFP at 22°C. Scale bars, 5 μm (left panels) and 10 μm (right panel). (C) Maximum projection of septin structures in wild-type A. gossypii expressing Cdc11a-GFP at 22°C. Scale bar, 5 μm. (D) Quantification of the relative abundance (based on sum fluorescence) of different septin structures in wild-type and cdc12-6 mutant Ashbya cells. Top, representative, cropped maximum projection images of Ashbya cells with color-coded localized septin structures (blue: IR rings; magenta: hyphal sides; purple: hyphal tips; orange: base of hyphal branches; white: needles). Septin structures were segmented from z-stacks images of an entire cell using the 3D Image Suite plugin in FIJI. Scale bar, 5 μm. Bottom, quantification of the sum fluorescence of different septin structures (as depicted in the above; structures ≥3 μm3) as a percentage of whole cell fluorescence above background in wild-type (blue bars) and cdc12-6 mutant (red bars) cells. N = 5 cells each. (E) Maximum projection of a cdc12-6-GFP A. gossypii mutant imaged at 22°C within 1 min after being shifted down from 37°C for 1 h. Scale bar, 10 μm. (F) TIRF images of wild-type (top) and cdc12-6 (bottom) septin filaments formed in solution at 250 nM on a PEG-coated coverslip. Cdc11-SNAP was fluorescently labeled with AlexaFluor 488 dye. Scale bar, 2 µm. (G) Persistence length as determined by the cosine correlation of tangent angles of filaments (wt, n = 37; cdc12-6, n = 66) polymerized in solution. Lines represent mean; bars represent range. (H) TIRF images of 3 nM wild-type and cdc12-6 septins (Cdc11-SNAP was fluorescently labeled with AlexaFluor 488 dye) polymerized into filaments on planar-supported lipid bilayers. Scale bar, 2 µm. (I) Model for Cdc12 AH domain function. Left, the Cdc12 AH domain enables septins to bind and distinguish micron-scale membrane curvatures. A cartoon depiction of the rod-shaped, nonpolar septin complex is capped with Cdc11 (yellow; CTE not included for clarity), Cdc12 (red shown with CTE), Cdc3 (purple with CTE), and Cdc10 (pink). Left inset, the full-length Cdc12 AH domain (blue) is at the C-terminus of the Cdc12 CTE (orange). Right, the absence of a fully functional Cdc12 AH domain promotes septin bundling in lieu of sensing membrane curvature. We speculate that absence of the AH domain leads to misfolding of the coiled-coil element of Cdc12, promoting coiled-coil interactions between Cdc3 subunits between septin complexes to promote bundling.