FIGURE 4.
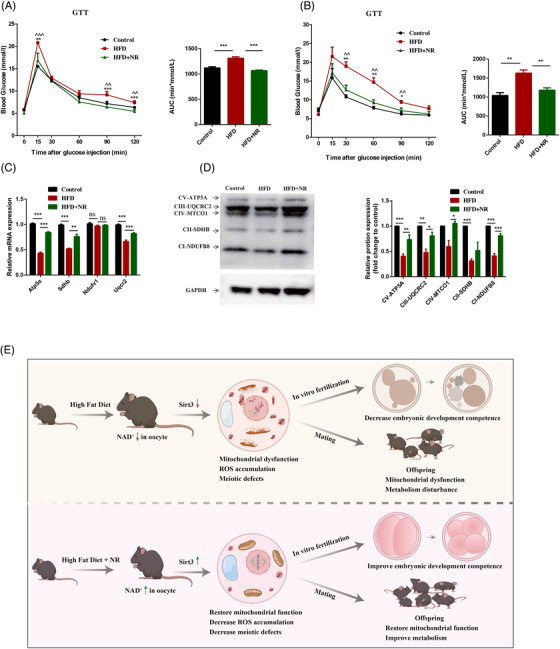
Sirt3 mediated protective effects of NR supplementation on metabolism and mitochondrial function in offspring. GTT of 7‐ to 8‐week‐old female (A) and male (B) mice born to HFD‐fed dams, HFD+NR‐fed dams, or HFD + NR fed dams (Sirt3 −/−), the area under curve (AUC) were also calculated based on the glucose levels of each time point. *Comparison between HFD and HFD+NR; ^Comparison of Control and HFD. (C) Transcription of genes related to OXPHOS in offspring's muscle from each group. (D) Immunoblotting for proteins related to electron transport chain complex content (CI, NDUFB8; CII, SDHB; CIII, UQCRC2; CIV, MTCO 1; CV, ATP5A). Relative expression of each protein was calculated as a ratio to GAPDH levels. (E) Diagram illustrating the beneficial effects of NR supplementation to ameroliate obesity‐induced oocyte dysfunction and improve offspring metabolism. Obesity decreases NAD+ levels in oocytes, which leads to mitochondrial dysfunction accompanied by ROS accumulation and meiotic defects, resulting in reduced oocyte quality and earlier embryonic developmental competence. NR supplementation restores mitochondrial functions in oocytes by restoring the NAD+ level and thus decreases ROS accumulation and meiotic defects via a Sirt3‐dependent pathway. Importantly, administration of NR attenuates the obesity‐induced decreases in early embryonic competence and the live birth rate as well as normalises the metabolism in offspring. * p < .05, ** p < .01, *** p < .001; ^^p < .01, ^^^p < .001
