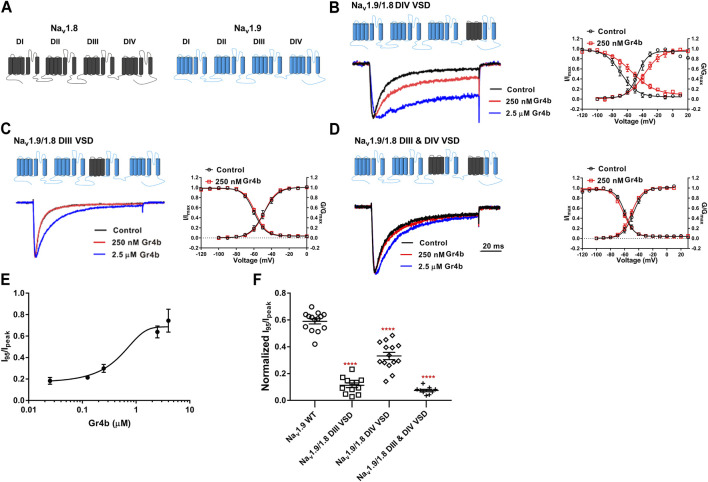
FIGURE 5.
DIII VSD and DIV VSD of Nav1.9 is involved in interacting with Gr4b. (A)The topological structure of Nav1.9 (blue) and rNav1.8 (grey), the numbers indicated the border of the transmembrane domains. (B–D) Representative normalized current traces, voltage-dependent steady-state activation and voltage-dependent steady-state inactivation show that the chimera channels (constructed as the cartoon illustrated) Nav1.9/1.8 DIV VSD, Nav1.9/1.8 DIII VSD, Nav1.9/1.8 DIII & DIV VSD, in the absence (black), presence of 250 nM Gr4b (red) or 2.5 μM Gr4b (blue). (E) The dose-response curves for the Gr4b-induced inhibition of the fast inactivation of Nav1.9/1.8 DIV VSD, EC50 of 1.5 ± 0.25 μM (n = 4). (F) The effect of Gr4b on the channel of WT and Nav1.9/1.8 chimeras. Dot plots display the effect of 250 nM Gr4b on the persistent current (I95/Ipeak) (n = 14 for WT; n = 12 for Nav1.9/1.8 DIII VSD, n = 14 for Nav1.9/1.8 DIV VSD and n = 9 for Nav1.9/1.8 DIII & DIV VSD). One-way ANOVA with Dunnett’s Multiple Comparison Test and compared with WT, **** p < 0.0001.